A test developed by OHSU researchers could help doctors treat cancer earlier when it's more likely to be cured.
Scientists discover plasma cell-free RNA profiling, which distinguishes cancers from premalignant conditions in solid and hematological malignancies. They developed a blood test to determine if a pre-cancerous condition is progressing to malignancy, potentially allowing therapy early in the tumor’s growth when cancer is more likely to be curable.
If the findings are confirmed in more extensive clinical trials, they could pave the way for a simple, low-cost technique of screening those at risk using a small blood sample taken a few times a year.
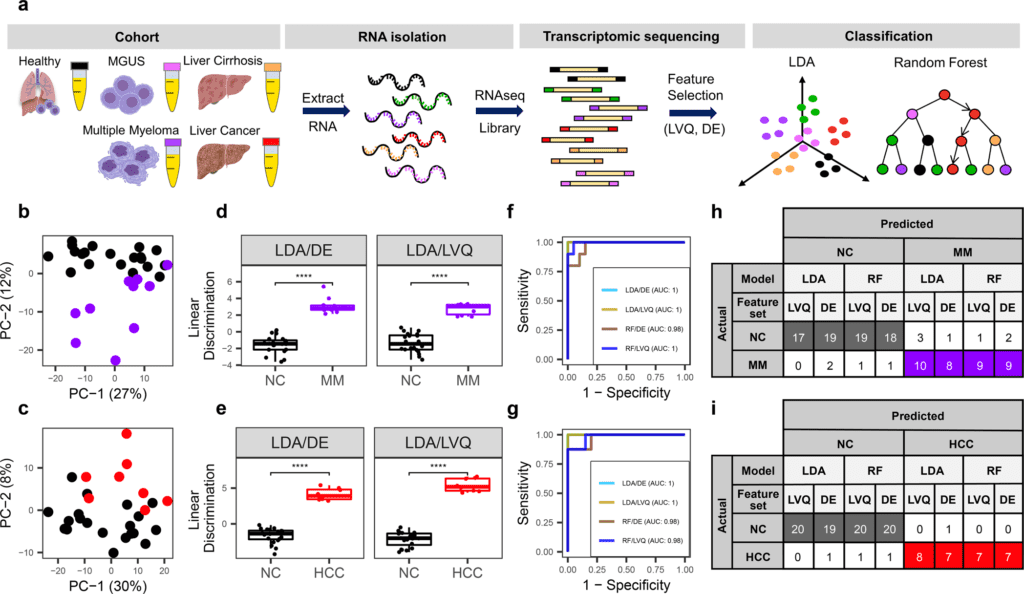
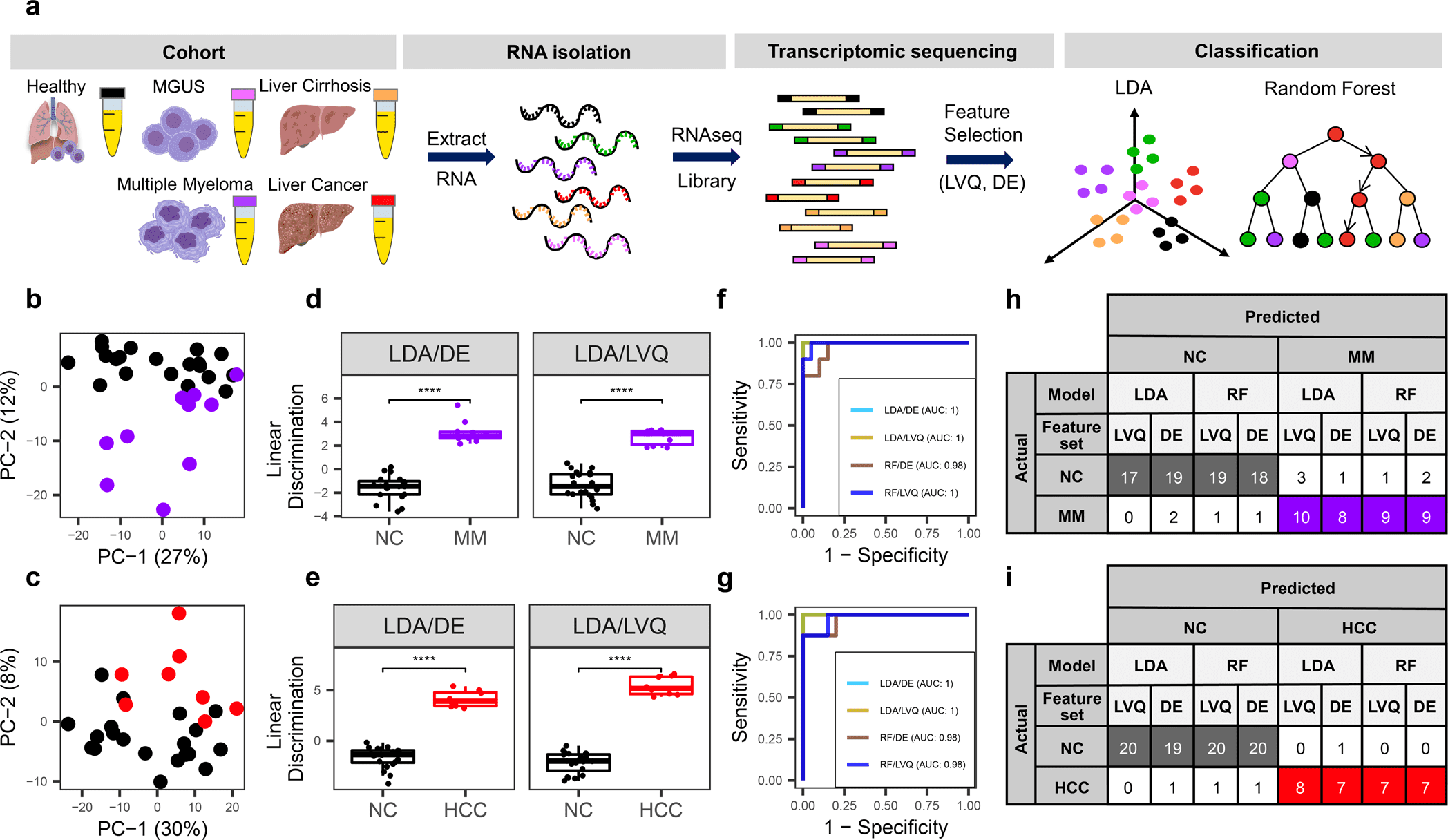
Image Source: Plasma cell-free RNA profiling distinguishes cancers from pre-malignant conditions in solid and hematologic malignancies
Cell-free RNA (cfRNA) in plasma reflects phenotypic alterations of both localized sites of cancer as well as the systemic host response.
Here, the scientists reported that cfRNA sequencing empowers the discovery of messenger RNA (mRNA) biomarkers in plasma with the tissue of origin-specific to cancer types and pre-cancerous conditions in both solid and hematologic malignancies.
Our biomarker may recapitulate the transition to cancer.
Thuy Ngo, Ph.D., lead author of the study and an assistant professor of molecular and medical genetics in the OHSU School of Medicine and a member of CEDAR, the OHSU Knight Cancer Institute’s Cancer Early Detection Advanced Research Center.
To investigate the diagnostic potential of total cfRNA from blood, the scientists sequenced plasma samples of eight hepatocellular carcinoma (HCC) and ten multiple myeloma (MM) patients, 12 patients with different pre-cancerous conditions, and 20 non-cancer (NC) donors.
The researchers identified distinct gene sets and built classification models utilizing Random Forest and linear discriminant analysis algorithms that could recognize cancer patients from premalignant conditions and NC people with high precision.
A few years ago, not many people believed cell-free messenger RNA could be reliably detected in the blood because it is prone to degradation. We found a way to handle it, and we are among the first to apply it in cancer and pre-cancer early detection.
Thuy Ngo, Ph.D.
Plasma cfRNA biomarkers of HCC are liver-explicit genes, and biomarkers of MM are profoundly expressed in the bone marrow contrasted with different tissues and are connected with cell cycle processes. The cfRNA level of these biomarkers showed a gradual transition from noncancerous states through pre-cancerous conditions and cancer.
Sequencing information was cross-validated by quantitative reverse transcription PCR, and cfRNA biomarkers were validated in an independent sample set (20 HCC, 9 MM, and 10 NC) with AUC greater than 0.86. cfRNA results seen in pre-cancerous conditions require further validation.
This work shows a proof of principle for involving mRNA transcripts in plasma with a plasma panel of genes to recognize cancers, noncancerous states, and pre-cancerous conditions.
Results of Advancements in Cancer Research
Although the latest advances in cancer research offer new techniques to treat cancer, early discovery of malignancy presents the most noteworthy possibility of working on long haul patient survival.
Presently, just 2.4% of metastatic liver cancer patients survive for more than five years. Early recognition of liver cancer, which has the most quickly expanding occurrence in the United States, can stretch out 5-year survival rates to 33% with current treatment options.
Indeed, even with hematologic malignancies like multiple myeloma (MM), 95% of patients are analyzed when cancer has spread systematically, coming about in essentially a 20% diminishing in 5-year survival rates contrasted with detection at earlier stages.
Non-invasive, minimal expense, and dependable cancer diagnostic assays could extraordinarily help patients by facilitating accessibility to early cancer screening.
There are disease states known to be precursors of malignant disease in numerous tumors. For instance, MM, a cancer of antibody-producing plasma cells, is in many cases preceded by monoclonal gammopathy of undetermined significance (MGUS), which is portrayed by lower levels of abnormal antibodies.
An Unmet Clinical Requirement for Simple Blood Tests
The predominance of MGUS is around 3% in the Caucasian populace, and the conversion rate from MGUS to multiple myeloma is ~1% per year. Hepatocellular carcinoma (HCC), the most prominent type of liver cancer, is many times preceded by liver cirrhosis (Cirr), characterized by irreversible fibrosis of the liver.
The prevalence of cirrhosis is between 4.5-9.5% of the worldwide population. The risk of developing de novo HCC in patients with liver cirrhosis ranges somewhere in the range of 1 and 5% each year, contingent upon the etiology of the cirrhosis.
To date, most early cancer recognition studies have zeroed in on distinguishing cancer from healthy controls instead of discriminating between cancer and common premalignant conditions.
In this manner, there is an unmet clinical requirement for a straightforward blood test that can recognize patients with premalignant conditions who require further intervention because of a higher probability of cancer incidence.
A cancer diagnosis is initiated with current clinical practices given expensive imaging studies or invasive screening systems.
On the other hand, a few cancers may simply stand ready with clinical symptoms present at further advanced stages. Fluid biopsy, a minimally invasive strategy for sampling and analyzing biomarkers in different body fluids, can potentially further develop cancer diagnosis and prognosis.
Fluid Biopsies
A few blood-based analytes have been investigated for use in fluid biopsies for cancer identification like circulating cells (circulating tumor cells (CTCs), circulating hybrid cells (CHCs), tumor-associated macrophages (TAMs)), circulating tumor DNA (ctDNA), platelets, and protein panels.
In any case, ctDNA and circulating cells are available at low levels, have different characteristics among patients, and weakly correlate with phenotypic changes in cancer.
Epigenetic features of ctDNA, for example, methylation and 5-hydroxymethylcytosine signatures, or ctDNA protected patterns, might give data about the tissue origin for pan-cancer detection.
Nonetheless, these techniques might require enormous deep sequencing coverage to be viable and may have insufficient sensitivity and specificity. Ongoing transcriptome analysis of tumor-educated platelets has shown guarantee pan-cancer detection, yet platelets are delicate, can be easily activated in vitro, and have exceptional variable characteristics depending upon their preparation which make them challenging to use with existing clinical blood tests.
Consequently, there is a requirement for robust fluid biopsy technology that can defeat these challenges in a safe, reliable, and cost-effective manner.
The Circulating Cell-Free RNA
Circulating cell-free RNA (cfRNA) in the blood is released from cells by active secretion or through apoptosis and necrosis.
Plasma cfRNA can potentially reflect the systemic response to developing tumors and give data about the tissue of tumor origin explicitly by cancer type. Past work has shown that global cfRNA profiles demonstrate temporal changes in organ-explicit transcripts.
Further investigation of these transcripts worked with the prediction of pregnancy delivery, preterm birth, and distinction of cancer from healthy controls. Here, they investigated the capability of cfRNA profiles to distinguish cancers from their premalignant conditions.
The scientists sequenced total plasma cfRNA from plasma samples of patients with HCC and MM and their pre-cancerous conditions, including liver cirrhosis (Cirr) and MGUS, and non-malignant growth (NC) donors.
They distinguished potential cfRNA biomarkers utilizing plasma cfRNA-sequencing of a pilot sample set and validated potential cfRNA biomarkers in an independent set.
The scientists further validated the sequencing information utilizing orthogonal measurement by quantitative reverse transcription PCR. Feature selection and classification models were built to investigate the capability of cfRNA profiles in separating malignant from premalignant conditions.
The Endpoint
In conclusion, the scientists proved that global profiling of cell-free mRNA could potentially lay out a stage for longitudinal monitoring of disease progression across solid and hematologic cancers.
This work establishes the groundwork for creating inexpensive assays that measure transcript levels of mRNA in plasma for a small panel of genes that can separate pan-cancer from premalignant conditions and generally healthy donors.
Intriguingly, organ-explicit enriched mRNA transcripts were distinguished as biomarkers that would demonstrate the tissue of origin for the tumor.
These cell-free plasma RNA biomarkers could be promptly joined with other nucleic acid-based and protein-based approaches for potentially increased diagnostic sensitivity and specificity.
You don’t need any fancy equipment for doing this. Any central lab with standard equipment can do the work. Sophisticated sequencing was only required for the discovery work of identifying the telltale gene signatures.
Thuy Ngo, Ph.D.
Article Source: Roskams-Hieter, B., Kim, H.J., Anur, P. et al. Plasma cell-free RNA profiling distinguishes cancers from pre-malignant conditions in solid and hematologic malignancies. npj Precis. Onc. 6, 28 (2022). https://doi.org/10.1038/s41698-022-00270-y https://news.ohsu.edu/2022/04/25/blood-test-could-reveal-transition-to-cancer-in-people-at-risk
Tanveen Kaur is a consulting intern at CBIRT, currently, she's pursuing post-graduation in Biotechnology from Shoolini University, Himachal Pradesh. Her interests primarily lay in researching the new advancements in the world of biotechnology and bioinformatics, having a dream of being one of the best researchers.