According to new research headed by University College London experts existing drugs that affect the impact of the hormone progesterone, such as mifepristone, have been found to reduce the risk of severe triple negative breast cancer in women with a BRCA1 gene mutation.
In premenopausal women, breast cancer is the main cause of death. Progesterone promotes the growth of luminal progenitor cells, which leads to the development of breast cancer with a bad prognosis.
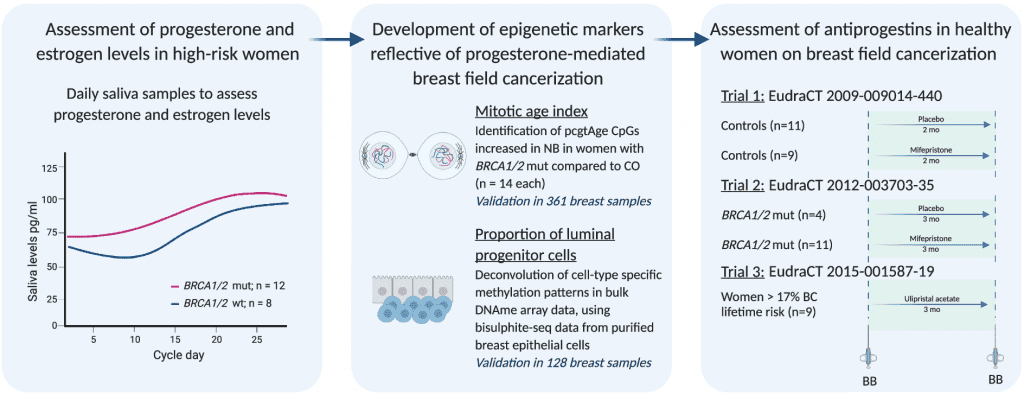
Image Source: Antiprogestins reduce epigenetic field cancerization in breast tissue of young healthy women
Triple negative breast cancer (TNBC) is found most commonly in women with the BRCA1 gene mutation, and it affects 13 out of every 100,000 women. TNBC patients have a worse prognosis because their malignancies are more aggressive and more likely to recur than other cancers. Furthermore, the cancer cells lack popular therapeutic targets (estrogen or progesterone receptors, as well as a protein called HER2), limiting treatment possibilities.
The scientists were interested to see if targeting progesterone signaling as a cancer prevention strategy was worthwhile by:
- Comparing progesterone levels during one menstrual cycle in women with and without a BRCA mutation.
- Developing markers for progesterone-mediated field cancerization in the breast.
- Testing the ability of progesterone receptor antagonists (mifepristone and ulipristal acetate) to reduce the level of field cancerization.
Scientists conducted a rigorous review of progesterone levels in women with a BRCA mutation and assessed the effect of medications targeting progesterone on the likelihood of getting breast cancer in women known to be at risk in early pilot research published in Genome Medicine.
Estrogen and progesterone levels were tested daily in 17 women (9 with a BRCA1 mutation and 8 without) using saliva samples. Researchers discovered that women with BRCA1 gene mutations had greater progesterone levels throughout the menstrual cycle, especially during the luteal phase. Higher levels of progesterone have been shown to increase breast cancer risk by indirectly acting on the cells that cause the tumor to grow faster by causing them to divide more frequently.
Researchers studied DNA methylation using cutting-edge technologies and discovered a ‘signature’ of markers in breast tissue (the WID-Breast29) that can signal and dynamically monitor progesterone-driven breast cancer risk.
The WID-Breast29 test was then performed on samples taken from the breast (a biopsy) of women who had taken part in a clinical trial of a progesterone-targeting medication, some of whom had a BRCA gene mutation. Mifepristone is a drug currently utilized in various therapeutic situations, including medical abortion, early miscarriage treatment, and Cushing’s Syndrome. It’s being tested as a regular contraceptive tablet right now.
Lower progesterone and cell turnover were detected in all of the women without a BRCA gene mutation and in 75% of the women with a BRCA gene alteration, indicating a lower breast cancer risk. In reality, some women with a BRCA gene mutation did not “react” to the drug by lowering their cancer risk, suggesting that they might need more preventive measures.
According to lead author of the study, Professor Martin Widschwendter (UCL EGA Institute for Women’s Health, Universität Innsbruck, Karolinska Institutet), breast tumours with the worst prognoses are linked to progesterone. The researchers measured progesterone levels every day for the duration of a menstrual cycle and found that progesterone levels are much greater in BRCA1 mutation carriers, who have a higher risk of developing breast cancer with a poor prognosis.
Most notably, the researchers demonstrated that medications that block progesterone function could minimize the cellular alterations that lead to cancer formation in young women’s normal breast tissue. The research team is quite enthused about the possibilities these findings have for better breast cancer prevention.
Women with a BRCA1 gene mutation have a 60-90 percent lifetime probability of having breast cancer, with 70% of those who do develop the more deadly “triple-negative” form. BRCA1 mutation carriers are currently offered a radical and invasive operation that involves removing both breasts to lower their cancer risk. A mastectomy, on the other hand, is a significant procedure with dangers and side effects such as infection and prolonged discomfort.
As a result, the researchers anticipate that, with more research, the WID-Breast29 test could one day be used to track women’s cancer risk over time and determine if medication interventions are helpful, allowing women to make more educated and personalized cancer risk reduction decisions.
A large-scale clinical trial will be required to see if progesterone modifying drugs combined with individualized treatment regimes and customized risk monitoring using the WID-Breast29 will reduce not only cancer cases but also avoidable surgical operations.
Everyone has heard of breast cancer and believe that treatment and outcomes have come on leaps and bounds in recent years. And that’s true with some breast cancer diagnoses – but not for the rarer and more aggressive forms of the disease.
Athena Lamnisos, CEO, The Eve Appeal
These new findings could be a game changer for what is currently a brutal diagnosis.
Story Source: Bartlett, T.E., Evans, I., Jones, A. et al. Antiprogestins reduce epigenetic field cancerization in breast tissue of young healthy women. Genome Med 14, 64 (2022). DOI: https://doi.org/10.1186/s13073-022-01063-5
https://www.ucl.ac.uk/news/2022/jun/progesterone-altering-drug-could-reduce-risk-aggressive-breast-cancer
Learn More About Bioinformatics:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.