Infectious diseases have been a persistent challenge to public health despite significant advancements in various scientific disciplines. The emergence of viruses resistant to drugs, the frequency of pathogen outbreaks, and the ongoing danger of pandemics underline the urgent need for new and effective treatments. Addressing these challenges requires the combined efforts of multiple disciplines, and recent artificial intelligence (AI) discoveries are proving to be game-changing. Systems biology, synthetic biology, and AI are opening the path for quick advancements in the fight against infectious diseases.
Artificial Intelligence’s Impact on Anti-Infective Drug Discovery
Drug-resistant microorganisms have caused anti-infective drug effectiveness to decline, highlighting the urgent need for cutting-edge therapies. AI, particularly machine learning (ML), has played a crucial role in facilitating the search for new drugs and repurposing existing ones. ML has been instrumental in virtually screening vast compound libraries, which would be impossible through traditional empirical methods. Additionally, AI integration has been particularly beneficial in the context of infectious diseases due to their phenotype-driven nature and the biological tractability of the pathogens involved.
Despite the advantages of AI in anti-infective drug discovery, several challenges remain. One major obstacle is the generalization of ML models to unexplored biomolecular spaces. To overcome this limitation, new approaches and models with improved inference capabilities, such as few-shot models and multitask models, are required to identify the most promising drug candidates. To reliably anticipate drug-target interactions and mechanisms of action, AI-driven drug development must also be combined with enhanced mechanistic models. Understanding drug interactions with membranes is crucial for developing effective drugs against Gram-negative bacteria.
Toxicity to host cells is a common concern in drug development, and ML models predicting toxicity have been hindered by the lack of high-quality datasets. Absorption, distribution, metabolism, and excretion (ADME) properties are also crucial for selecting suitable drug candidates. However, predicting efficacy in animal models of acute systemic infections remains a challenging task not yet addressed by ML-driven approaches.
To advance anti-infective drug discovery, a combination of experimental and computational approaches is necessary to address predictive power and data scarcity. ML models that incorporate information from scarce training data and leverage extensive search spaces are likely to enhance drug discovery significantly. Generative ML models will continue to propose chemical structures and peptide sequences for synthesis and evaluation. Integrating disparate scientific information through AI platforms can improve our understanding of biology and chemistry, guiding experimental methods to augment search spaces. Interpretable or explainable ML approaches can help infer important structural features and improve model learning from data.
Artificial Intelligence driven Insights into Host-Pathogen Interactions
Understanding host-pathogen interactions and immune responses is essential for developing effective treatments and vaccines for infectious diseases. This field has advanced significantly thanks to AI, systems biology, and synthetic biology.
ML models, including random forest classifiers and complex language models, have been applied to predict immunogenicity, evaluate pathogen virulence, and guide the development of vaccines and therapeutic drugs. Reverse vaccinology, which relies on immunologic and genomic information, has been facilitated by supervised ML approaches. ML has made significant contributions to analyzing large and complex datasets in infectious disease research, but challenges persist in understanding host-pathogen interactions and immune responses.
Addressing these challenges requires integrating high-throughput datasets with detailed mechanistic studies and experimentation. Comprehensive datasets acquired across different infection contexts can foster the development of AI models capable of making generalizable hypotheses and inferences. Sequence-based ML approaches, guided by biological sequences or chemical structures, offer tunable approaches to investigating infection biology.
ML has also been applied to process microscopy datasets, leading to insights into host-pathogen biology. Furthermore, ML plays a role in vaccine development by accelerating design and predictions from computational models. However, challenges in data quality, availability, and validation need to be addressed to enhance the predictive power of ML in vaccine development.
ML has informed personalized antibiotic recommendations in clinical settings and can aid in clinical decision-making for infection management. To improve the application of ML to infection-related contexts, future work aims to make biology more “embeddable,” represented by low-dimensional features like sequences, vectors, or graphs. Integrating ML with next-generation systems and synthetic biology methods will drive progress and facilitate a better understanding of host-pathogen interactions, antigen selection, vaccine design, and treatment strategies.
Application of Artificial Intelligence in Diagnosis and Synthetic Biology
Timely and accurate diagnostics are vital for controlling the spread of infectious diseases. AI has greatly enhanced diagnostic capacities in conjunction with synthetic biology, gene expression analysis, mass spectrometry, and imaging.
Various synthetic biology approaches, such as enzymatic reactions, toehold switches, and CRISPR-Cas enzymes, have been successfully used for detecting diseases like malaria, Ebola, Zika, and COVID-19. ML has played a crucial role in designing these biomolecules, with neural networks being commonly used to model the data.
Mass spectrometry, imaging-based diagnostics, and synthetic biology have all benefited from the use of ML. The adoption of these methods has substantially boosted antimicrobial susceptibility testing, which is crucial for establishing how anti-infective drugs should be administered. However, challenges remain, such as data quality for new pathogens, the limited generalizability of current approaches, and the need for highly accurate diagnostic predictions in clinical settings.
The availability of high-quality data, potential biases in ML models, and the severity of consequences in the case of inaccurate diagnostic predictions are key challenges in applying ML to diagnosis. It’s important to address these issues to ensure reliable and equitable diagnostics.
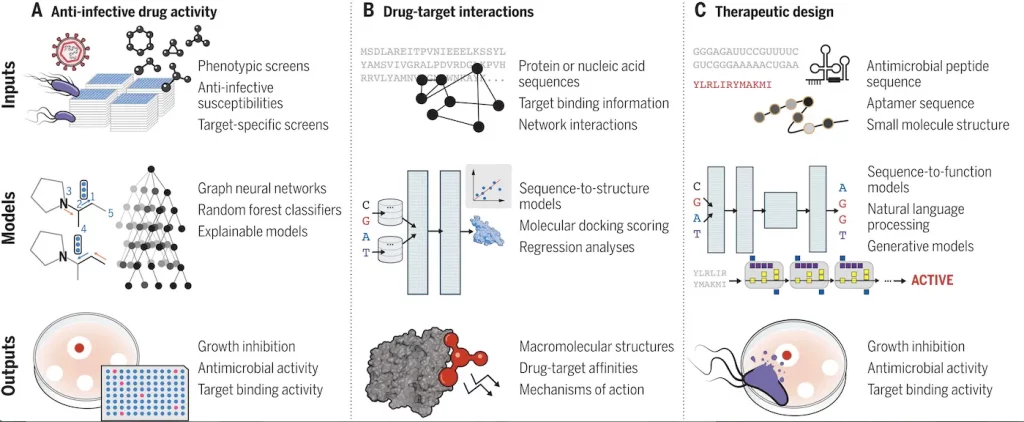
Image Source: https://doi.org/10.1126/science.adh1114
The creation of ML models that deliver precise diagnoses in clinical settings, the application of AI to clinical and field-deployable diagnostics, and the ML-guided design of synthetic circuits for low-cost and portable diagnostics will be the main research areas in the future. Sequence-to-function and generative models are promising areas for growth. ML models must be optimized, evaluated for biases, and trained on robust data to achieve high accuracy.
Conclusion
Artificial intelligence, coupled with systems and synthetic biology, is transforming the fight against infectious diseases. Its application in anti-infective drug discovery, infection biology, and diagnostics has shown great promise, significantly advancing our understanding and control of infectious diseases.
To fully harness the potential of AI, interdisciplinary collaboration and the generation of comprehensive datasets are essential. Advancements in AI models’ accuracy, interpretability, and generalizability will enable safer and more effective drug development, improved infection biology research, and highly accurate diagnostics.
Looking ahead, the integration of AI with systems and synthetic biology will continue to drive innovation in infectious disease management. As AI technologies mature, unprecedented progress is expected in combating infectious diseases and safeguarding public health worldwide. The potential for AI to lead the charge in eliminating infectious diseases and averting pandemics in the future is enormous.
Story Source: Reference Paper
Learn More:
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.









