The emergence of mRNA vaccine technology represents a monumental leap forward in disease prevention. A review article published in Nature Reviews Drug Discovery by researchers at the University of Pennsylvania and Carnegie Mellon University discusses the evolution of messenger RNA (mRNA) based vaccines over the course of the past few years. The powerful effects of mRNA vaccines were exhibited during the COVID-19 pandemic in 2020. These vaccines were one of the most widely produced and administered vaccines across the globe to combat the SARS-CoV-2 retrovirus that was spreading rapidly at the time, and are the most popular known example of mRNA vaccines. The review emphasizes the potential mRNA vaccines hold for viruses beyond the one that caused the 2020 pandemic, along with alternative delivery modes to viral modes, such as those based on lipid particles.
Importance of vaccines
The administration of vaccines is a necessity to tackle life-threatening diseases as well as to increase the average lifespan of the general population. They reduce fatality rates usually caused by highly infectious diseases like polio, smallpox, influenza, and tetanus. They provide us with protection against these diseases by inducing the generation of antibodies that are specific to the pathogen present in the human body, enabling the individual’s body to fight the disease when it strikes in the future.
Traditional vaccines have failed to deal with pathogens like human immunodeficiency viruses (HIV, the causative organism for AIDS), Plasmodium falciparum (the parasite that causes malaria), and hepatitis C. Vaccinations for influenza require regular upgrades and modifications due to the rapidly evolving nature of the genome of the influenza virus.
Introducing mRNA vaccines
mRNA vaccines have been developed to be a safer and cheaper alternative to traditional vaccines over the last three decades. Traditional vaccines incorporate inactivated particles of viral RNA directly into the human genome to induce the production of antibodies by the immune system. However, this methodology of administration has raised concerns regarding the possibility of induced insertional mutagenesis within the genome. mRNA vaccines eliminate this possibility as they are not integrated into the genome at all.
Here are a few advantages of mRNA vaccines:
- The production of mRNA vaccines is cheaper compared to traditional vaccines, thus, it is more cost-effective.
- It has better scalability and decreased duration of production, with increased productivity; a single bioreactor can produce over a million mRNA vaccines in one go.
- The application of mRNA vaccines can be extended to other antigens as well, as it provides an increased intensity of immune response.
- These vaccines account for various mutations that can take place within the genome of the pathogen, with their dose being regulated accordingly.
On listing out all these benefits, you may consider, ‘Why were mRNA vaccines not the norm beforehand when they offer so many benefits to the industry and general public?’ The answer to that question lies in the fact that scientists and medics were initially skeptical of mRNA vaccines when they were first discovered, as they believed these vaccines to be unstable with poor efficacy, and they also raised concerns over the possibility of these vaccines stimulating an excessive immune response, which can have health consequences for the patient receiving the vaccination. Despite all of the scrutiny, a few companies and biotech-based startups continued to vouch for the production of these vaccines, and it is thanks to them that we were able to deal with the COVID-19 pandemic with fewer casualties than initially estimated by epidemiologists. There is ongoing research into the regulation of immunostimulation by mRNA vaccines by performing studies and experiments on their pharmacology.
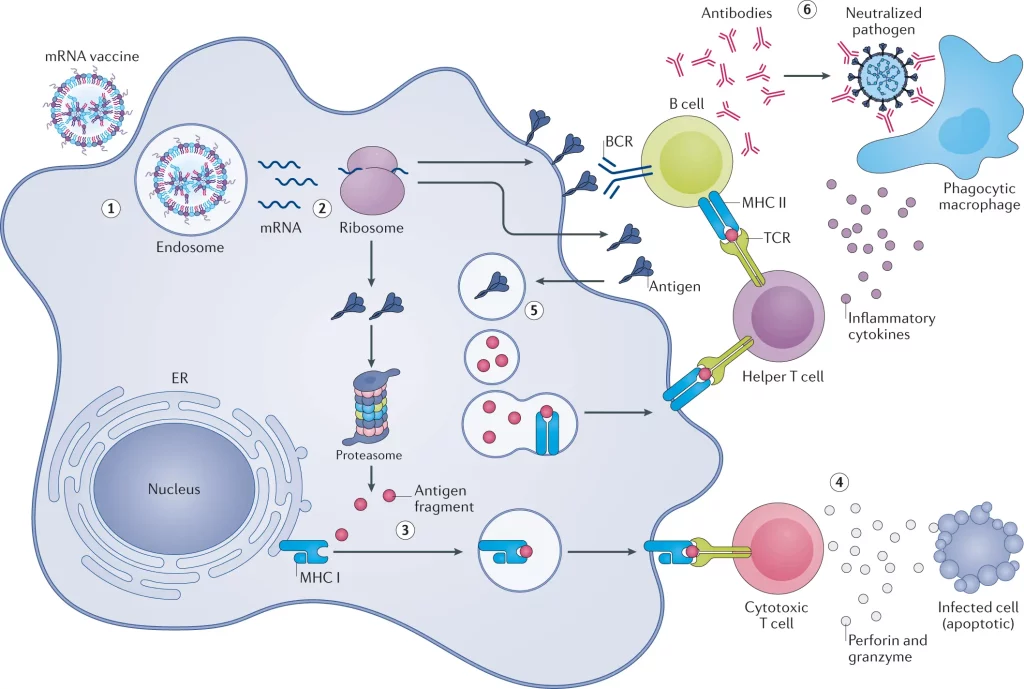
Image Source: https://doi.org/10.1038/s41573-021-00283-5
Delivery of mRNA vaccines
mRNA vaccines, on their own, are incapable of infiltrating the lipid bilayer that outlines the cell membrane, as it provides a barrier to the entry of foreign particles into the cell. The cell membrane is semi-permeable and only allows specific molecules to enter the cell’s cytoplasm. It is anionic in nature, while the mRNA is negatively charged, leading to repulsion between the two.
mRNA is also vulnerable to being consumed by the cells of the immune system and to degradation by nucleases present within the cell. Techniques like employing a gene gun and electroporation have been used to facilitate the intracellular delivery of mRNA.
Lipid-based nanoparticles (LNPs)
One of the most recent innovative methods of transfecting immune cells with mRNA is by using nanoparticles based on lipids for transporting it. This method has been used for most vaccinations administered during the COVID-19 pandemic.
Some of the benefits of LNPs are:
- They can be easily modulated
- Compatible with different kinds of biological molecules
- It can be formulated with ease
- Have a large capacity for holding mRNA molecules
They consist of four major components:
- Cholesterol, a steroid
- Lipids that are ionizable
- Phospholipids, which act as helper molecules
- PEGylated lipids: They cover the delicate core of the mRNA within a capsule and protect it.
One of the first lipids to deliver mRNA successfully in 1989 was DOTMA, with DOTAP as its synthetic analog. DOTMA is a positively charged lipid that encapsulates the negatively charged mRNA. This gets rid of the repulsion faced by mRNA molecules when it’s near the surface of the cell membrane. Another example of cationic lipids used for mRNA delivery in the past is Lipofectamine. The only downside to cationic lipids is that they trigger pro-inflammatory immune responses, which is an inconvenience to the patients to whom the vaccine is being administered.
Ionizable lipids have been developed as a promising alternative to cationic lipids. When injected into the bloodstream, they are neutral in terms of charge. This makes them safer and increases the duration of the time they spend in circulation within the bloodstream, in comparison to cationic lipids. A combination of lipids and mRNA is incorporated into nanoparticles for transportation. The positive charge of this delivery system facilitates its fusion into the endosomal membrane, therefore granting direct access to the cytoplasm. DODAP and DODMA are the first examples of delivery systems of this kind, with later developments that led to the production of DLin-MC3-DMA. It can also deliver small interfering RNA (siRNA) and patisiran (Onpattro).
Conclusion
mRNA vaccines have the potential to curb any future pandemics that may be on the rise and have successfully brought pandemics such as the one in 2020 under control in a formidable manner. Their success has accelerated research and development into LNPs and RNA therapeutic techniques, elevating their status from niche instances to being administered to large populations. These vaccines can revolutionize cancer immunotherapy and protein replacement therapy.
Article source: Reference Paper
Learn More:
Swasti is a scientific writing intern at CBIRT with a passion for research and development. She is pursuing BTech in Biotechnology from Vellore Institute of Technology, Vellore. Her interests deeply lie in exploring the rapidly growing and integrated sectors of bioinformatics, cancer informatics, and computational biology, with a special emphasis on cancer biology and immunological studies. She aims to introduce and invest the readers of her articles to the exciting developments bioinformatics has to offer in biological research today.