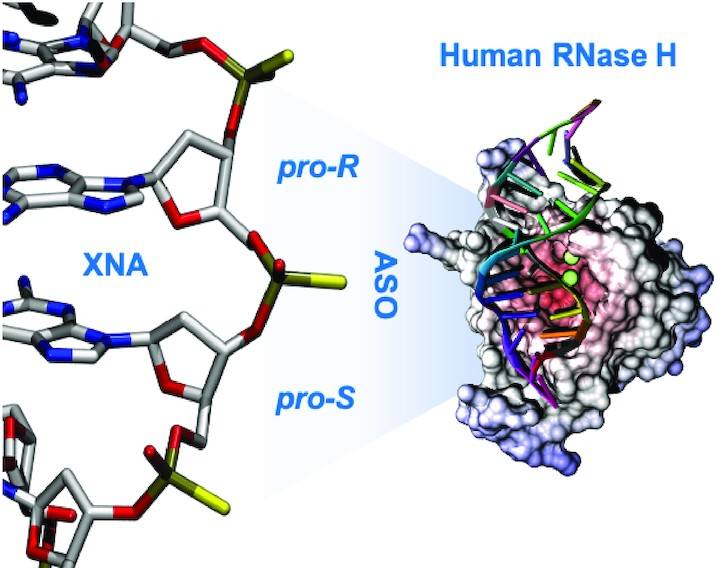
A consortium of scientists from across the globe has undertaken a massive computational effort to produce predictive models on the effect of phosphorothioate (PS) stereochemistry on the structure and stability of the PS-DNA.RNA hybrid in the realm of gene silencing. Phosphorothioates have proven significant in oligonucleotide-based cancer and neurological disease therapeutics. PS-oligonucleotides have acquired a fundamental place in gene silencing-based therapeutics due to the increased nuclease resistance upon introducing PS substitution for antisense oligonucleotides (PS-ASOs). However, there exists a need for more knowledge pertaining to the effect of PS stereochemistry on DNA.RNA hybrids. The authors performed computational experiments aided by experimental measurements using NMR spectroscopy, thermodynamic analysis, and MD simulations to develop predictive models for a better understanding of this.
What are antisense oligonucleotides?
Antisense oligonucleotides (ASOs) are short single-stranded polymers designed to interact with RNA molecules, thereby affecting RNA functionality. They have been widely used to generate a therapeutic response in gene silencing-based disease management. Several of these have received FDA and EMA approval for treating various severe pathologies, and dozens more are expected to hit the market soon. The biological mechanisms related to ASO pharmacology are as follows: steric blockage of ribosomal binding, splice-switching, and RNAse H-mediated degradation of target RNAs.
In the degradation of RNA using ASO, RNase H recognizes the ASO.RNA complex degrades the RNA strand, leaving behind the ASO intact to capture another molecule of RNA and start another functional cycle. Several studies have shown that ASO should be based on a DNA backbone to generate well-structured DNA.RNA hybrid, although DNA-based ASO produces strong DNA.RNA complexes, however, the single-strand DNA quickly degrades. Thus, therapeutic applications require the DNA strand to be well protected against degradation. This involves the application of protecting carriers as well as DNA backbone modifications. Of these, the most effective mechanism is the substitution of one or more of the phosphates of DNA with a phosphorothioate (PS) derivative. This substitution does not affect the DNA.RNA structure as well as its thermostability. It has also been shown to increase nucleic acid lipophilicity as well for better cellular delivery and is also well tolerated by RNase H.
Challenges in the ASO-based gene silencing therapeutics
Phosphorothioates (PS) are chiral (Sp and Rp stereoisomers), and this can create a racemic mixture. Due to this, specific chiral effects on the ASO and ASO: RNA topology cannot be evaluated.
The impact of PS on the biological activity of DNA.RNA complex has been studied since the ’90s. However, such studies have resulted in controversial outcomes. Earlier studies suggested that PS-DNA.RNA hybrids are susceptible to RNase H degradation, however, opposite results were found in recent studies.
Similarly, early NMR and CD studies indicated small topological changes in the PS-substituted DNA.RNA complex, whereas recent NMR studies have shown that the flexibility of the hybrid is compromised.
Also, structural studies of stereo-pure PS hybrids are sparse and limited to pure Sp or Rp hybrids.
Thus, establishing clear rules on the connection between chirality and ASO’s activity is crucial and could improve ASO-based therapeutics by leaps and bounds.
The massive Computational effort and the simulation tools
The authors combined structural studies and state-of-the-art simulation tools to develop a guideline for the design of chirality-controlled PS-based ASOs. In order to do so, they performed computational experiments to study the impact of PS chirality on the topology and mechanics of DNA.RNA hybrids performed MD simulations of the DNA.RNA duplexes. The studies also revealed the essential dynamics of PS hybrids, along with PS chirality and thermostability. The authors also explored the impact of PS on enzymatic activity. Overall, the authors were successful in laying the groundwork for clear rules pertaining to the connection between chirality and PS-based ASO bioactivity, thereby generating a therapeutic approach that could potentially address a wide variety of severe diseases involving the overexpression of proteins as well as the expression of aberrant proteins.
Conclusion
The authors show that current computational technologies and simulation toolkits could be utilized for investigating the oligonucleotide’s ability to form stable hybrids, resist serum nucleases, and lead to hybrids sensitive to RNase H degradation, with reasonable accuracy and also find differences associated with small changes in the backbone, such as in the R/S chirality of phosphate substitutions. In the future, the authors aim to validate their results in cell cultures and further strengthen the optimal guideline for targeting a variety of DNA.RNA hybrids, thereby addressing many more diseases. The small size of these molecules, coupled with their cost-effectiveness, has made these molecules the favorites in the drug discovery community, and with the current guidelines presented by the authors, PS-based ASO therapeutics will certainly be a significant choice of therapeutics for several diseases.
Article Source: Reference Paper | Reference Article
Learn More:
Banhita is a consulting scientific writing intern at CBIRT. She's a mathematician turned bioinformatician. She has gained valuable experience in this field of bioinformatics while working at esteemed institutions like KTH, Sweden, and NCBS, Bangalore. Banhita holds a Master's degree in Mathematics from the prestigious IIT Madras, as well as the University of Western Ontario in Canada. She's is deeply passionate about scientific writing, making her an invaluable asset to any research team.