Researchers from University Hospital Frankfurt and Goethe University Frankfurt have figured out how bacteria attach to host cells, paving the way to create a new class of antibiotics.
(Photo Credit)
Image Source: https://aktuelles.uni-frankfurt.de/englisch/how-bacteria-adhere-to-cells-basis-for-the-development-of-a-new-class-of-antibiotics/
The initial and most significant step in the development of infectious diseases is always the attachment of bacteria to host cells. Infectious pathogens use this adhesion first to colonize their host organism, which in this case is the human body, and then to start an infection that, in the worst-case scenario, could be fatal. Finding therapeutic alternatives that obstruct this vital relationship in the initial stage of infection requires a precise understanding of how the bacteria adhere to host cells.
Our understanding of the molecular mechanisms behind bacterial adherence, which is a crucial stage in infection, can be considerably improved by investigating the intricate interactions between adhesins and host cell receptors. Since Fn is so widely distributed in the pericellular environment, bacteria binding to Fn may be an intriguing technique for colonization. Bacteria cling to host cells in a variety of ways.
Critical interaction of trimeric autotransporter adhesins with the human protein fibronectin
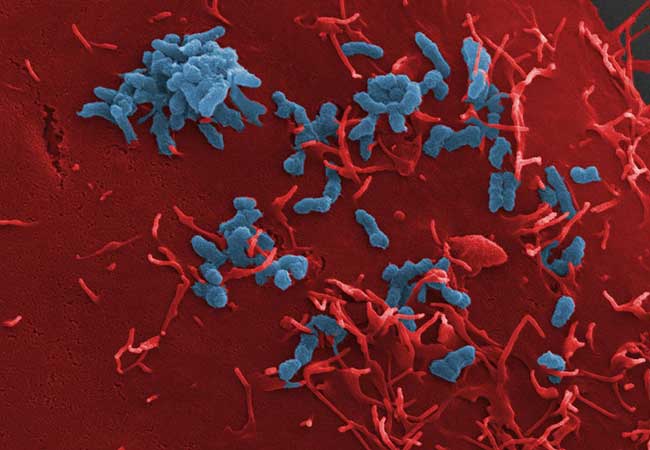
The human-pathogenic bacterium Bartonella henselae has been used by scientists from University Hospital Frankfurt and Goethe University Frankfurt to explain the precise bacterial adhesion process. The “cat-scratch disease,” which is passed from cats to humans, is brought on by this infection. Using a mix of in-vitro adhesion tests and high-throughput proteomics, the bacterial adhesion process was elucidated in an international collaborative initiative directed by the Frankfurt research group led by Professor Volkhard Kempf. The study of all the proteins found in a cell or a complex organism is known as proteomics.
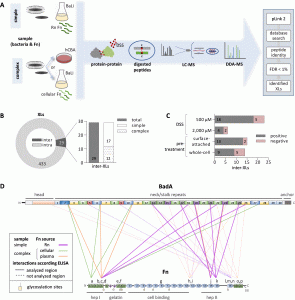
Image Source: https://aktuelles.uni-frankfurt.de/englisch/how-bacteria-adhere-to-cells-basis-for-the-development-of-a-new-class-of-antibiotics/
The researchers have revealed a crucial mechanism: the interaction of a certain class of adhesins, known as “trimeric autotransporter adhesins,” with fibronectin, a protein frequently found in human tissue, causes bacteria to adhere to host cells. Adhesins are components on bacteria’s surface that help the pathogen stick to the biological structures of the host. Many other human-pathogenic bacteria, including the multi-resistant Acinetobacter baumannii, which the World Health Organization (WHO) has designated as the top priority for research into novel antibiotics, contain homologs of the adhesin that has been found here as being crucial.
Modern protein analytics were used to visualize the precise sites of protein interaction. It was also demonstrated that experimental inhibition of these mechanisms virtually eliminates bacterial adherence. Therapeutic strategies that aim to contain bacterial adhesion in this way could represent a promising alternative treatment as a new class of antibiotics (known as “anti-ligands”) in the continuously extending domain of multi-resistant bacteria.
The scientific work was accepted as “Paper of the Month” by the German Society for Hygiene and Microbiology (DGHM) on 18 June 2022 after being published in the esteemed journal “Microbiology Spectrum” of the American Society of Microbiology (ASM).
The implication of the study
In this study, the researchers show that host-fibronectin and TAA both play essential roles in bacterial adherence and give the precise arrangement of interconnected amino acids from each protein. This research demonstrates the domain-specific pattern of interaction between TAA and fibronectin to adhere to host cells and offers a potential strategy for preventing bacterial infections by preventing bacterial adhesion, which is typically the initial stage of infection.
J., V. D., Arno, T., S., L. M., Johan, M., Dirk, L., A., E. J., … M., A. J. (2022). Interaction of Bartonella henselae with Fibronectin Represents the Molecular Basis for Adhesion to Host Cells. Microbiology Spectrum, 10(3), e00598-22. https://doi.org/10.1128/spectrum.00598-22 https://aktuelles.uni-frankfurt.de/englisch/how-bacteria-adhere-to-cells-basis-for-the-development-of-a-new-class-of-antibiotics/
Learn More About Bioinformatics:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.





