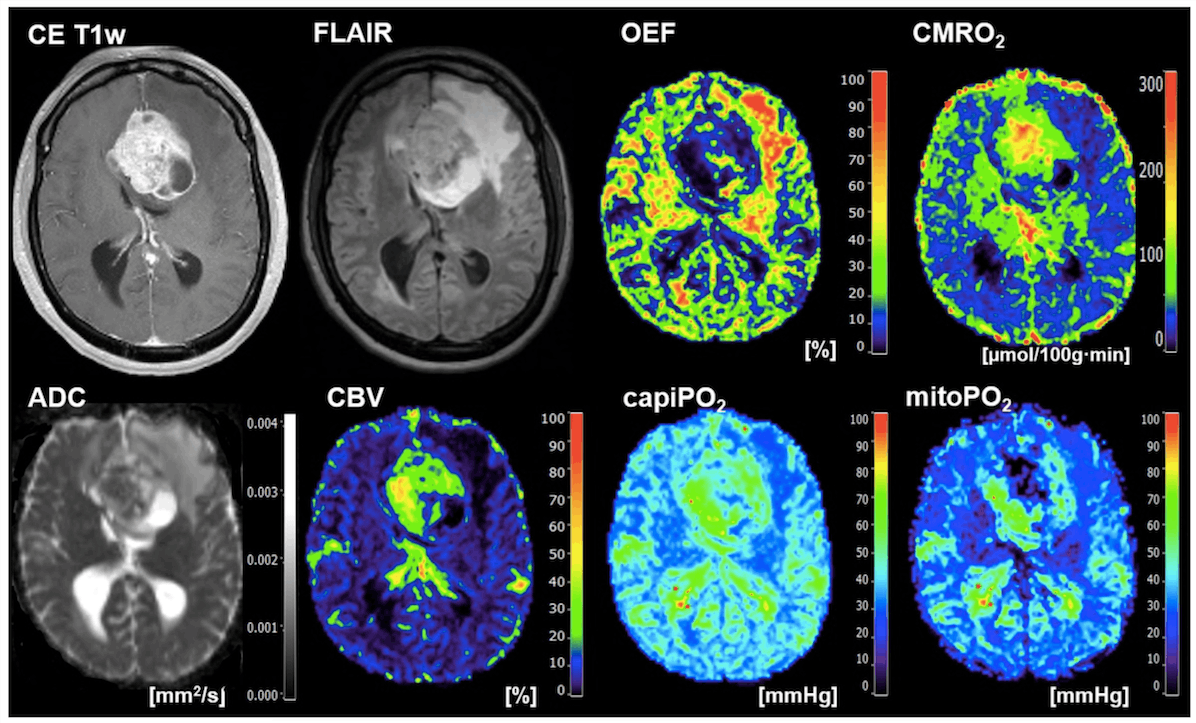
According to a recent study from Karl Landsteiner University of Health Sciences (KL Krems), using radiomics and deep learning algorithms can quickly and accurately distinguish between glioblastoma (primary tumors) and brain metastases. The study, which was published in Metabolites, discovered that magnetic resonance-based radiological data of tumor oxygen metabolism provide a solid foundation for discrimination via neural networks. This combination of oxygen metabolic radiomics and AI analysis was discovered to be vastly superior to human expert evaluations in all critical criteria, even when essential oxygen values did not differ significantly between tumor types. The neural networks’ ability to make clear distinctions based on these values demonstrates the method’s potential.
Glioblastoma (GB) and brain metastasis (BM) are the most commonly occurring types of brain tumors in adults. While they may appear similar on traditional MRI scans, they are treated very differently. Accurately identifying a patient’s tumor type can have a significant impact on their outcome. Using new metabolic neuroimaging techniques in conjunction with artificial intelligence has the potential to improve diagnostic accuracy while also making implementation easier in clinical practice. However, this method produces a large amount of data that is difficult to handle in routine diagnostic evaluations.
To address the issue, the researchers proposed a DL-based method for assessing radiomic feature vectors from MR-based oxygen metabolism data in order to pre-therapeutically distinguish between glioblastoma and brain metastasis. This is intended to help radiologists and clinicians make timely diagnoses and treatment decisions.
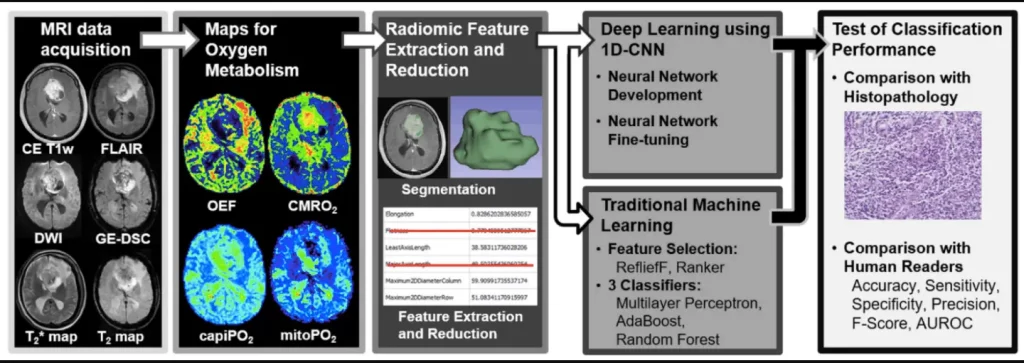
Image Source: https://doi.org/10.3390/metabo12121264
The Model or Radiologist?
The model proved to be more successful in distinguishing between tumor types than even human specialists could. Radiologists were outperformed by this technique in all critical differentiation metrics, including accuracy, sensitivity, specificity, and precision. Furthermore, statistical measures such as F-values and AUROC were found to be more effective than human evaluations.
The measurements were based on a “Convolutional Neural Network” (CNN) that the team created. This is a type of artificial neural network that mimics parts of biological processes and is designed for machine learning and the processing of image or audio data. As part of the study, the CNN was trained using tumor data from St. Pölten University Hospital’s extensive database and then used to analyze the MR-based oxygen levels of new patients.
Diagnostic Potential of Deep Learning and Radiomics
The study collected data on brain oxygen utilization, “cerebral oxygen turnover” (CMRO2) and oxygen levels in the mitochondria (mitoPO2), which provide insight into cellular energy production. Although the average and middle values of these parameters did not vary significantly between the two types of tumors, the deep learning algorithm was able to distinguish between them.
The study demonstrates the tremendous diagnostic potential of combining the two methods. However, in everyday clinical practice, radiological data on O2 metabolism are still used to a very limited extent. The research team would like to change this, and they are already planning a more extensive study that will not only confirm the current data but will also use methods that are closer to clinical practice. Some manual steps were still required in the current study to prepare the data for analysis, which is very time-consuming for clinical routine and also limits institutional comparability. As a result, the researchers intend to use CNN in this phase as well.
Conclusion
The research investigates the feasibility and utility of radiomic features derived from MRI-based oxygen metabolism (oxygen metabolic radiomics) in conjunction with a deep learning algorithm (1D-CNN) for pre-therapeutic differentiation between glioblastoma and brain metastasis, the most common and difficult-to-distinguish types of contrast-enhancing brain tumors in adults. Although mean and median CMRO2 and PO2 values did not differ significantly between tumor entities, the radiomic properties of these oxygen-metabolic biomarkers were particularly well suited for classification using deep learning.
Article Source: Reference Paper | Reference Article
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.