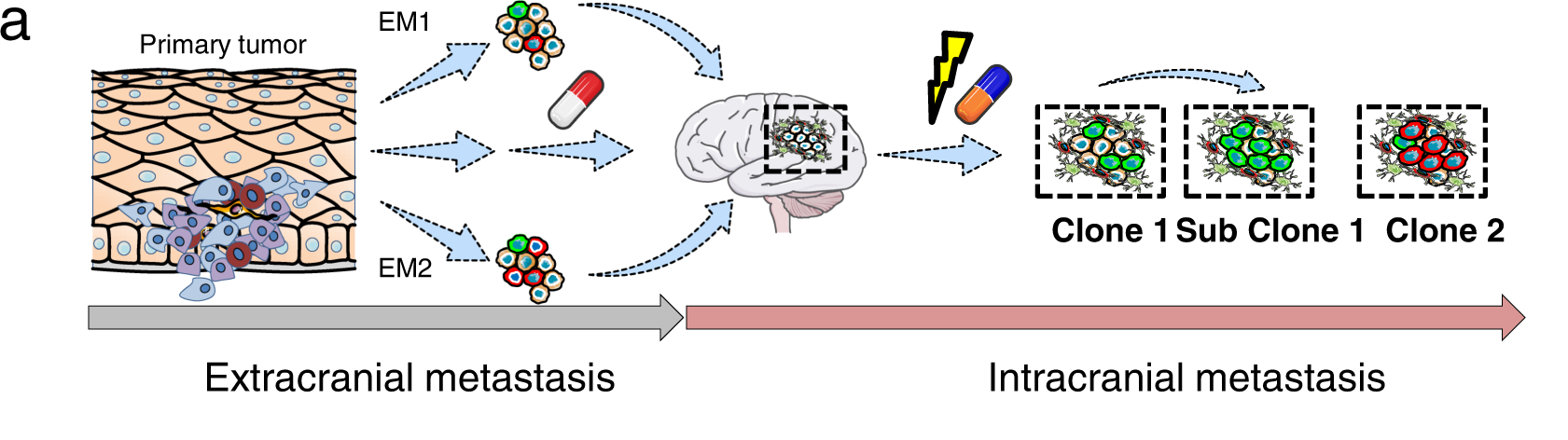
Researchers used a multi-OMICS technique and targeted sequencing (TargetSeq) to identify the processes that may govern the progression of brain metastases. Regardless of intracranial location, it was found that the expression of E-cadherin (Ecad) or Nerve Growth Factor Receptor (NGFR) progresses tumors into proliferative and therapy-resistant states. The investigation of MAPK inhibitor-naive and refractory melanoma brain metastases (MBM) demonstrates that Ecad-associated processes transition to NGFR-associated processes during progression. A global methylome analysis reveals 46 different methylated sites that mutated MBM from wild-type MBM.
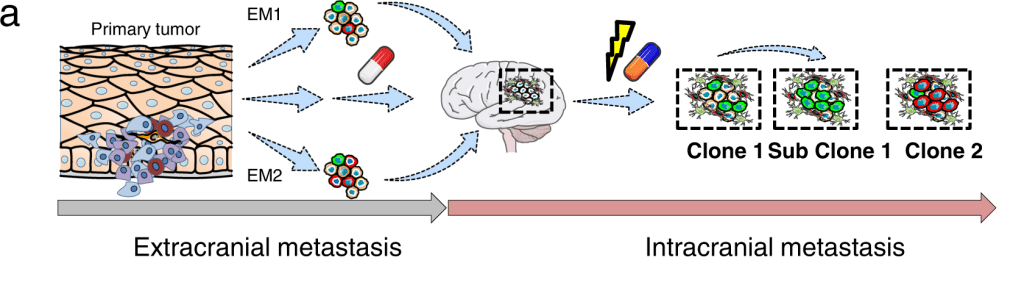
Image Source: https://doi.org/10.1038/s41467-022-34899-x
Brain metastases are commonly observed in melanoma, lung, and breast cancer. Despite several progress and remarkable responses observed in a subset of patients, the immune checkpoint inhibitors (ICi) block oncogenic BRAF (BRAFi) or interfere with the PDL1/PD1 axis to restore T cell activation are deemed to be insufficient for long-term prevention of intracranial relapse and progression.
MBM develops in 20-40% of melanoma patients along the course of the disease, resulting in an 8.9-month overall survival after MBM identification. The interval between primary tumor diagnosis and identifying MBM ranges from 1 to 10 years, implying a gradual evolution of MBM from circulating tumor cells that breach the blood-brain barrier (BBB).
The primary functions of melanoma cells involve survival, migration, and stemness are maintained by NGFR protein, which in turn is also linked to metastasis, drug resistance, and cellular plasticity. Cellular plasticity allows melanoma cells to transition between various states, and regulating NGFR expression levels controls proliferation and invasiveness.
In the skin, NGFR governs the migration of melanocytes by E-cadherin (Ecad)-mediated adhesive junctions. The downregulation of Ecad is required for melanocyte migration and malignant transformation state. It is rarely plausible to follow the exact cellular subclones that give rise to MBM in human patients. Thus to address it, the authors present evidence that at least two distinct molecular stages of MBM formation and progression are defined by Ecad and NGFR expression.
Major Findings
Most melanoma patients have poor prognoses because of intracranial progression, interlinked with neuroinflammation and the development of multiple brain metastases. Brain metastases, in particular, are formed by molecular processes that are poorly understood, which might involve a founder clone giving rise to numerous subclones; or the transformation of dormant micrometastases into active macrometastases triggered by the microenvironment or therapeutic interventions.
A proliferative drug-naive or drug-responsible phenotype was present in Ecadhigh MBM, and the grouping of tumors is likely dependent on oxidative phosphorylation (OXPHOS). OXPHOS was downregulated in NGFR+ MBMs, showing that they belong to a different subclass of tumors than Ecadhigh MBM. It was discovered that NGFR+ melanoma cells are in charge of intracranial relapse and progression and might manifest under hypoxic settings.
Although Radke et al. discovered a mutually exclusive pattern of gene expression for Ecad- and NGFR-correlated genes, the cellular assays suggest that Ecad and NGFR are indeed interconnected via cellular plasticity. As a result, melanoma cells that are Ecad+ can become NGFR+ cells, and vice versa. The findings imply that Ecad+ phenotypes i) Lost but regained at distant organ sites like the brain, ii) Or retained during metastasis, and can be re-acquired by melanoma cells.
The timing and course of intracranial advancement can be affected by the interaction of tumor cells with other glial cells, such as astrocytes, microglia, and tumor-infiltrating lymphocytes (TILs). A high TIL status of MBM is typically linked to favorable survival. But it was found that NGFR was highly expressed in TlLhigh tumors, indicating no direct link, at least in MBM. The study is limited because only a small sample size was considered, with the results pointing to a T cell triggering a dedifferentiation process in TlLhigh/NGFR+ MBM.
Final Thoughts
In conclusion, the study shows that both Ecad and NGFR-controlled programs divide MBM at the molecular level, thus indicating the existence of at least two distinct cell states that may respond differently to therapeutic interventions. The Ecad-to-NGFR transition likely acts as a milestone in developing MBM and temporally separates tumors into those that progress versus those that do not. However, this study’s relative scarcity of malignancies results from difficulty accessing MBM. As a result, more research is needed to thoroughly understand the molecular mechanisms behind the genesis and development of single and multiple brain metastases in melanoma and other malignancies with a high prevalence of brain metastases, such as lung and breast cancer.
Article Source: Reference Paper
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Shwetha is a consulting scientific content writing intern at CBIRT. She has completed her Master’s in biotechnology at the Indian Institute of Technology, Hyderabad, with nearly two years of research experience in cellular biology and cell signaling. She is passionate about science communication, cancer biology, and everything that strikes her curiosity!