Scientists from Purdue University unravel cell migration direction under the influence of environmental cues using a ternary logic gate system. Cells detect several environmental cues and process intracellular signals to determine the direction of migration. Cell migration is involved in various physiological mechanisms as well as pathological processes. Signaling pathways and molecules involved in directed cell migration have been identified, but the integrated influence of multiple chemical and fluidic cues in determining the direction remains elusive. The authors reverse-engineered a cancer cell’s migratory behavior and built a cellular signal processing system as a ternary logic gate.
Cell migration and signaling: the knowns and unknowns
Directed cell migration drives several biological processes, such as embryonic development, cancer metastasis, wound healing, inflammation, and angiogenesis. Cells are subjected to numerous heterogeneous environmental cues during directed migration. These cues typically include chemical, mechanical, and fluidic cues. For instance, cancer cells have been shown to have a biased migratory direction when induced by chemokines or growth factors such as TGF-β. Interstitial fluid has also been shown to play a significant role in directed cell migration. What still remains elusive is how cells integrate simultaneous cues to determine the direction of migration.
Several studies involving chemical or fluidic cues induced directed cell migration have identified key signaling molecules. Chemical cues are typically sensed by the cells as concentration gradients of chemokines or growth factors via corresponding receptors on the surface of the cells. These receptors include G-protein coupled receptors (GPCR) and receptor tyrosine kinases (RTK).
Upon receiving the signals from the cues, cells transduce these cues to a migratory signal through intracellular signaling pathways to carry out the directed migration. It has been shown that GTPases are involved in directed cell movement and are locally activated by RTKs.
It has been shown that fluidic cues also result in directed cell movement by activation of focal adhesion kinases ( FAK) through integrin, ERK, and PI3K. It was reported that cell trajectories almost aligned with the flow streamlines upon FAK activation.
Quantitative biophysical aspects of directed cell migration have also been studied. Biochemical relations of the ligand-receptor binding kinetics have been used in analyzing cellular sensory precision for the detection of shallow chemical gradients. To better understand the cellular sensing and processing machinery, models have been developed for the spatial and temporal variations in the diffusion and activation parameters of the intracellular signaling molecules.
Given the advancement in understanding signaling regulated directed cell migration, what still remains a puzzle is how cells determine the direction of migration under several simultaneous heterogeneous cues, like chemical and fluidic cues. Previous studies have looked at scenarios involving multiple chemical cues, as in cancer cells being exposed to two kinds of growth factors.
Multiple cues of a similar kind have been shown to have synergistic as well as antagonistic effects in the determination of the direction of cell migration. For the first time, the authors studied the impact of simultaneous heterogeneous cues, chemical and fluidic, on directed cell migration.
The ternary logic gate
In order to understand how cells decide on the direction of migration under simultaneous heterogeneous cues, the authors first created a cellular microenvironment with controlled chemical and fluidic cues. They used a microfluidic platform having a center and two channels on the sides. The center has embedded cells in a collagen mixture, and the side channels are the source and sink filled with medium. Manipulation of chemical concentration and pressure variances between source and sink results in the simultaneous chemical gradient and pressure-driven flow.
Parallel flow is represented as ‘+,’ a positive direction to the chemical gradient, and counter flow is represented as ‘-,’ a negative direction to the chemical gradient. The authors use this engineered cell setting to understand the effect of simultaneous heterogeneous environmental cues on directed cell migration.
The authors first investigate the extracellular coupling of the heterogeneous cues-chemical and fluidic. They consider two specific scenarios, viz, parallel flow as an additive cue with the chemical gradient and second counter flow as a competing cue to the chemical gradient. The chemical cue, TGF-β, coupled with the fluidic cue, is next exposed to a murine pancreatic cancer cell line, KIC. With the engineered cell set-up, the authors venture out to characterize directed cell migration.
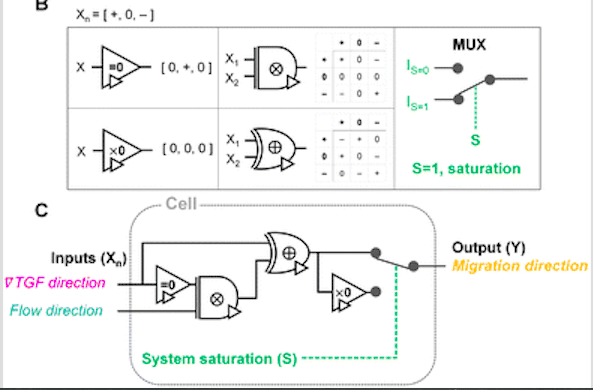
The authors find that the chemical cue is lowered below the cellular sensing criterion in regions created due to extracellular combinations of the chemical and fluidic cues, indicating a 0-state. They find that the cells effectively select a chemical cue to process mixed signals of chemical and fluidic cues. The authors report that when cells can detect chemical and fluidic cues, they tend to follow a chemical gradient direction under the additive combination with the parallel flow and the competitive combination with the counter flow. The cellular response was seen to be completely unbiased when the processing capacity was saturated. With these observations in mind, the authors proposed a framework for the cellular sensing machinery using a ternary logic gate as follows in the figure.
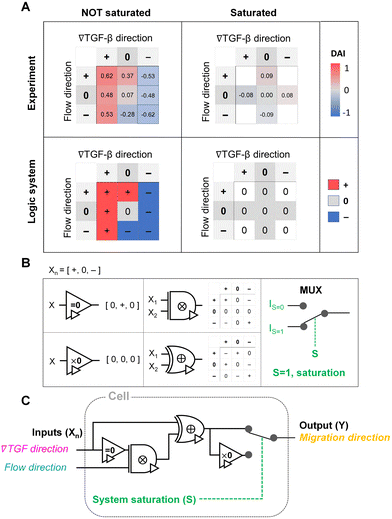
Image source: https://doi.org/10.1039/D2LC00807F
Conclusion
In this study, the authors present a framework to understand how cells decipher multiple environmental cues, chemical and fluidic, to determine the direction of cell migration by proposing a ternary logic gate circuit. The results, as well as the proposed logic circuit, demonstrate that the shared pathway of the chemical and fluidic cues determines directed cell migration. The proposed logic gate circuit could be a blueprint for future endeavors in synthesizing functional signaling processing machinery for engineered cells which could be used in therapeutics and drug delivery.
Article Source: Reference Paper | Reference Article
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Banhita is a consulting scientific writing intern at CBIRT. She's a mathematician turned bioinformatician. She has gained valuable experience in this field of bioinformatics while working at esteemed institutions like KTH, Sweden, and NCBS, Bangalore. Banhita holds a Master's degree in Mathematics from the prestigious IIT Madras, as well as the University of Western Ontario in Canada. She's is deeply passionate about scientific writing, making her an invaluable asset to any research team.