Artificial intelligence (AI) and cellular biology have combined to change several sectors, including the mapping and monitoring of cellular activity within the human body, as technology continues to improve at an unprecedented rate. In a quest for a deeper understanding of conditions such as dementia and infectious diseases like COVID-19, scientists at IMB applied artificial intelligence to develop a novel 3D blueprint of critical cellular constituents.
A team of researchers from IMB (Institute for Molecular Bioscience at the University of Queensland) and the University of Bristol created a model of the 16-subunit Commander complex, a collection of proteins that serve as cells’ “postal workers.” The research findings were published this week in the journal Cell.
According to the authors, the intricate system within our bodily cells functions akin to the procedures observed within a postal system, facilitating the transportation and organization of proteins.
Role of Commander Complex in Infection, Alzheimer’s, and Heart Diseases
Efficiently transporting cargo relies on the precise delivery of suitable bundles to their designated destinations at the appropriate time. Likewise, within cellular realms, the Commander complex orchestrates this intricate mechanism, ensuring the precise amount of protein is conveyed to its intended station. This intricate protein transportation system is believed to influence various ailments such as heart disease, Alzheimer’s disease, and infections.
Uncovering the Path to Drug Discovery through Structural Insights
Gaining insight into the intricate structures of these proteins enables us to grasp their functioning mechanisms, fathom the reasons behind disease-causing mutations, and devise future-oriented therapeutic approaches to target them specifically.
The Commander complex plays a crucial role in the life cycles of viruses such as SARS-CoV-2, the culprit behind COVID-19, and the human papillomavirus (HPV), a notorious instigator of cancer. Additionally, this complex has been implicated in the intricate task of ferrying the amyloid protein in Alzheimer’s disease.
Individuals affected by the rare Ritscher-Schinzel syndrome, marked by cognitive limitations and a delay in growth, experience elevated levels of cholesterol and irregularities in the heart due to mutations in the Commander complex. These genetic alterations impact the movement of lipids into cells, leading to the observed abnormalities.
Advancements in Technology Lead to a Deeper Understanding
Deeper insights into the intricate framework of the Commander complex will help in understanding its functioning, thereby amplifying our knowledge of its role in disease progression.
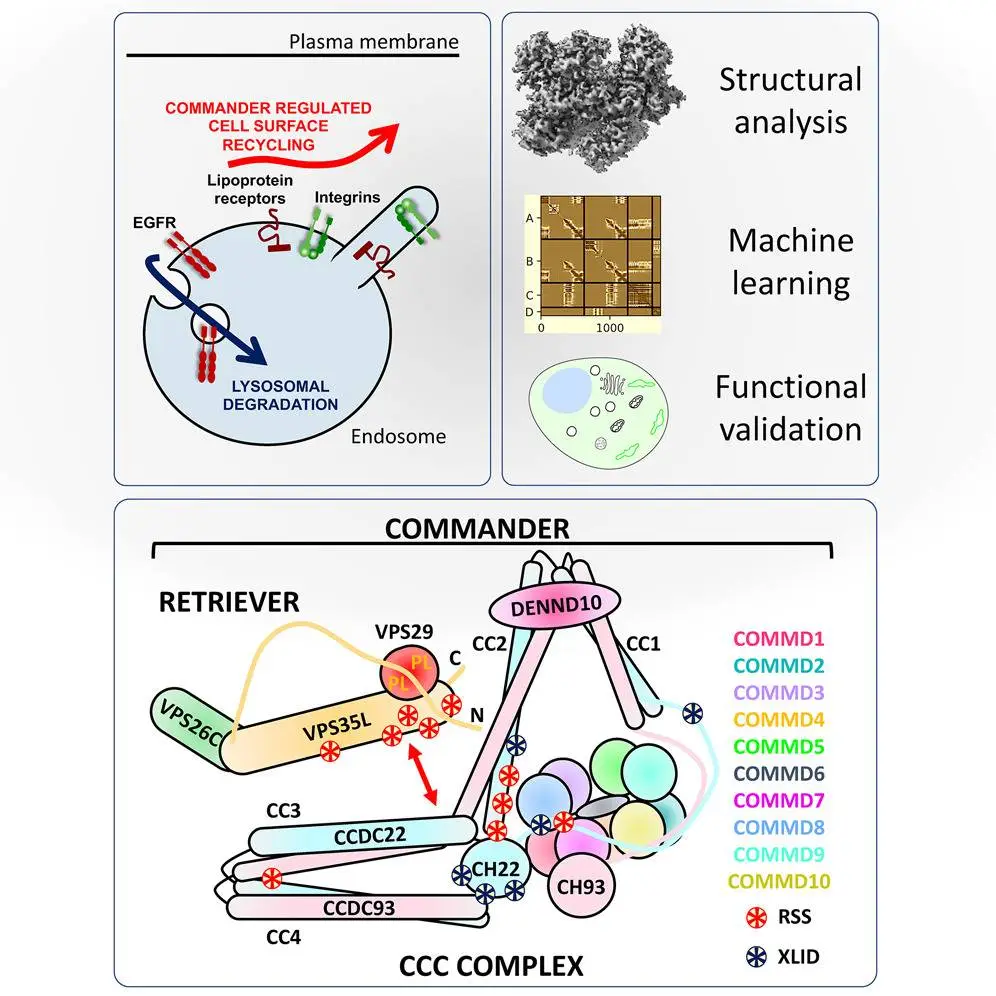
Utilizing state-of-the-art electron imaging and advanced machine learning methods, the multinational team disassembled the entire assemblage of the Commander protein complex into its elemental constituents.
According to the authors, before the development of these cutting-edge AI technologies a few years ago, the complex design of the Commander complex would have remained a mystery.
Limitations of the study
The research offers a structural framework for analyzing Commander function in various cellular and disease-related processes. Although scientists have determined the core structures of the Commander complex, certain aspects, such as the coiled-coil regions and the complete atomic structure of Retriever, remain unresolved. Additionally, studying the full sixteen subunit Commander holo-complex will be crucial for a comprehensive understanding of their organization and assembly on the endosomal membrane.
Conclusion
The Commander complex is crucial for recycling transmembrane cargo in the endosomal system and is implicated in Ritscher-Schinzel syndrome. A comprehensive structural model of the Commander complex was developed through a combination of X-ray crystallography, electron cryomicroscopy, and computational modeling. The structure enables the identification of disease-causing mutations and unveils the molecular features essential for the function of this evolutionarily conserved trafficking machinery.
Article Source: Reference Paper | Reference Article
Learn More:
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.