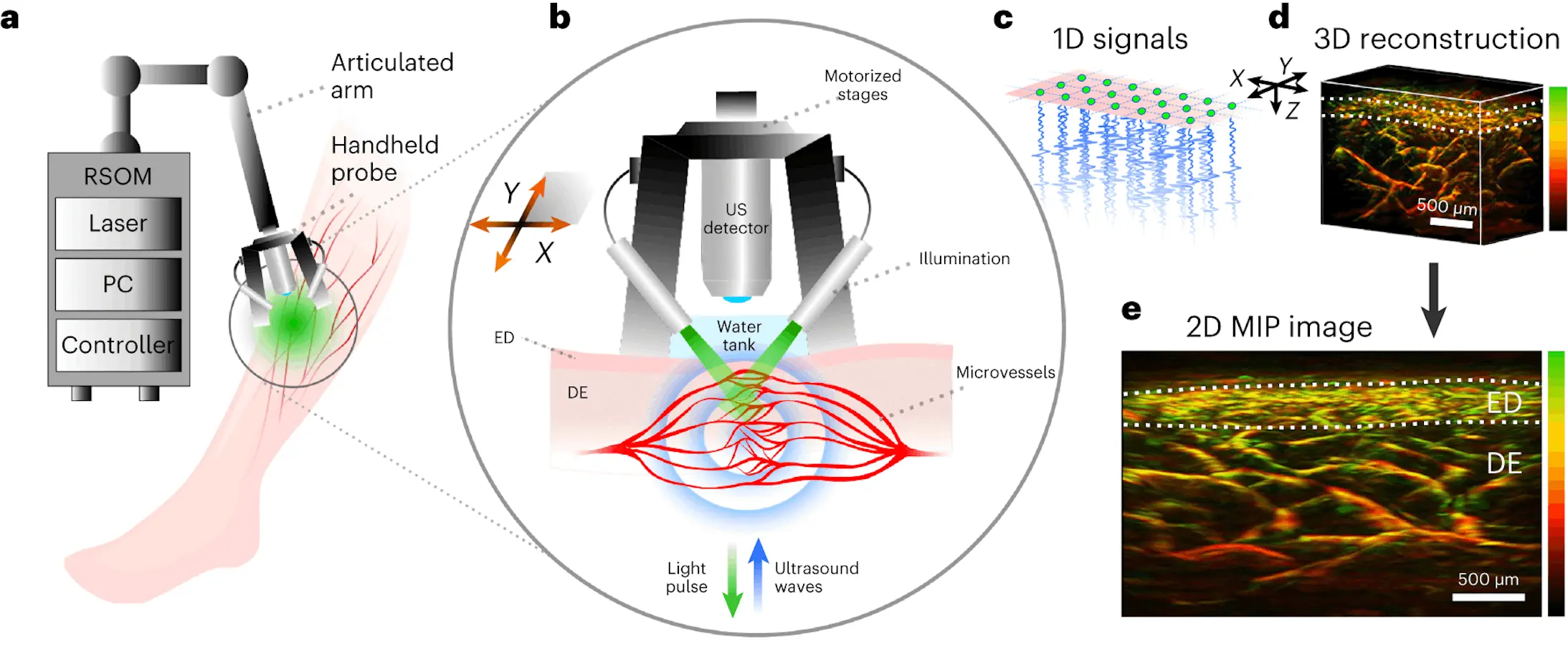
A new non-invasive imaging technique called raster-scan optoacoustic mesoscopy (RSOM) shows promise for detecting diabetes progression and severity by visualizing microscopic changes in the skin’s blood vessels. Recently published research from the Technical University of Munich (TUM) and Helmholtz Munich reveals RSOM’s ability to create detailed three-dimensional maps of the skin’s microvasculature down to capillary dimensions. Machine learning then pinpoints subtle alterations reflecting vascular damage tied to advancing diabetes.
Tracking disease progression in diabetes remains a major challenge. Current blood tests like glycated hemoglobin (HbA1c) analysis lack sensitivity for detecting early vascular complications or monitoring subtle worsening over time. Skin manifestations also tend to appear late and crudely indicate systemic microvascular damage. This often delays interventions until irreversible organ injury sets in.
The new technique leverages the skin’s accessibility to “read out” diabetes’ impacts on tiny vessels in ways not previously possible. It images living tissue without dyes or contrast agents using optical and ultrasonic waves. Powerful AI methods then extract and analyze hundreds of explainable microvascular features predictive of diabetes severity.
Studying Microvascular Architectural Changes
The researchers imaged a 4x2x2 mm3 skin volume on participants’ lower legs using RSOM’s high-resolution capability. The technique detects hemoglobin as an endogenous contrast agent, visualizing the intricate vascular network across skin layers. Machine learning segmentation algorithms isolated dermal and sub-epidermal vasculature to enable precise 3D quantification of the micro-anatomy.
In total, 64 structural features were computed describing vessel density, branching, sizes, shapes, and orientations at different scales – from tiny <100 μm capillaries to large feeding vessels. These parameters sensitively capture diabetes’ impacts on the cutaneous vascular tree in ways never before quantified.
The analysis included 115 participants, with 75 having type 1 or 2 diabetes manifestations ranging from no complications to advanced vascular disease. Forty healthy individuals formed a control group.
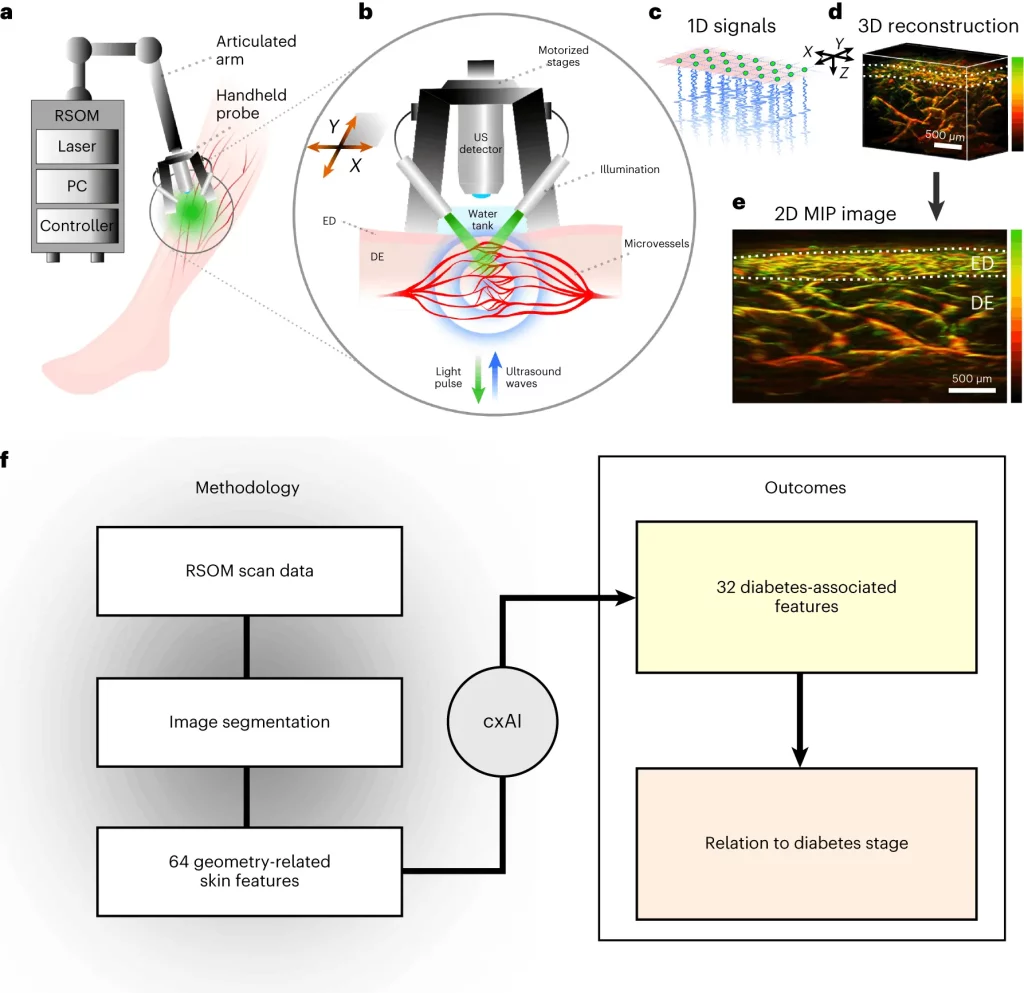
Image Source: https://doi.org/10.1038/s41551-023-01151-w
Microvascular Insights: Diabetic Degeneration in Dermal Layer
Excitingly, the study revealed substantial diabetic microvascular degeneration manifesting prominently in the vital skin layer. The number of tiny junction-to-junction vessel branches significantly decreased, indicating network rarefaction and chronic ischemia.
Conversely, vascular features increased in the upper sub-epidermal layer, possibly representing a reactive response. This dichotomy explains inconsistencies in past histology studies, which are limited to isolated biopsies providing 2D snapshots needing more depth resolution.
Notably, the middle “mesoscale” features probing vascular architecture from 100-1000 μm showed the highest diabetes association. This level likely indicates incipient pathology better than the smallest capillaries or macro vessels.
AI Model Predicts Diabetes Progression Stage
The research further developed an AI model aggregating 32 optimized features into a “microangiopathy score,” quantifying vascular change severity. The score differentiated healthy controls from diabetes with excellent accuracy (0.84 AUC). It also sensitively classified patients into three complication levels spanning none to advanced vascular disease.
The microangiopathy score remained a significant predictor even after accounting for potential age confounding. Younger patients showed similar skin deterioration compared to older people when matched by diabetes duration, metabolic control, and complications.
Study Implications
This breakthrough investigation establishes RSOM imaging of the skin microvasculature as a promising new technique for precision diabetes diagnostics and monitoring.
The detailed insights into microscopic vascular damage mechanisms also open intriguing research avenues. Extending the approach to probe specific diabetes subtypes and therapies could accelerate treatment advances.
Most exciting is the prospect of a rapid, non-invasive test allowing frequent tracking of vascular health over time in the clinic. This could genuinely personalize and optimize care, detecting subtle deterioration years before it becomes irreversible or symptomatic. The skin may offer the ideal biopsy-free window into the systemic microcirculation’s dynamic responses to diabetes and interventions.
Conclusion
This innovative study establishes non-invasive imaging of the skin’s microvasculature as a promising new technique for detecting diabetes progression through detailed mapping and analysis of subtle vascular alterations reflecting systemic damage.
Story Source: Reference Paper | Reference Article
Learn More:
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.