The experience of pain is complicated, subjective, and unique to each person. While some people have a high pain tolerance, others may regard even minor discomfort as excruciating pain. Pain affects over 1.5 billion people worldwide and costs more than $600 billion in the United States annually. Despite the availability of various pain relief medications, not all types of pain can be cured. In addition, drugs that alleviate pain may cause dependency and tolerance, particularly in the scenario of opioids like morphine. Understanding the mechanisms of pain can help us develop better pain management treatments.
The researchers have identified a gene mutation that may regulate pain. The study, reported in the Journal of Clinical Investigation, found that TRPV1, which is a sensory neuron receptor that captures noxious stimuli, including heat and the burning sensation of chili peppers, has a potential pain insensitivity mutation in the gene that encodes this protein.
The study is a collaborative effort by Münster University Hospital in Germany, Stanford University, and Emory University in the United States. The study’s analysis of human mutations was aided by a prior understanding of birds, which, unlike mammals, have a TRPV1 receptor that is naturally resistant to noxious insults and even spicy food but still perceives pain. Birds also have a number of mutations that are common in humans.
The TRPV1 channel and pain perception
There are over 1,000 different TRPV1 mutations in humans. Although attempting to deactivate the receptor to alleviate pain is not a novel idea, previous attempts have failed. However, there have been recent advances in this field.
Initially, a number of medications produced through this procedure disrupt the regulation of body temperature. Furthermore, TRPV1 plays an important role in transmitting the sensation of warmth, and altering its operation completely eliminates the physical discomfort, obstructing the perception of scorching heat that serves as a safeguard.
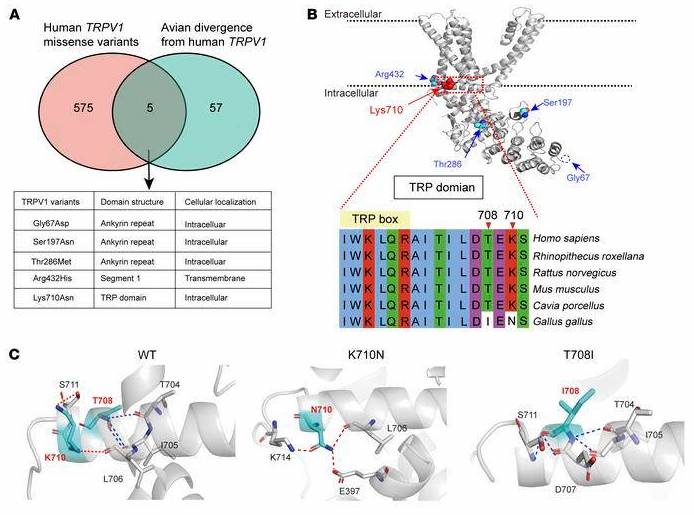
To begin their investigation, the scientists looked through a genetic information database to see how the genetic sequences of human and avian TRPV1 compared. Using a computational technique, they identified five avian mutations that they believe are linked to pain tolerance.
Cryogenic electron microscopy can visualize structures with near-atomic resolution without requiring large sample sizes or crystallization. Using this method, scientists discovered five avian mutations in the amino acid residue K710, which is thought to control the opening and closing of the TRPV1 channel.
The mutations can be found in humans as well, but they are extremely rare, so the researchers decided to see what results would appear if they were “transplanted” into mammals. When these variants were tested in genetically modified cells, they observed that the function of the channel was indeed altered. The researchers then used the CRISPR/Cas9 gene editing technology to create mice with the mutation K710N, which had previously been shown to reduce the receptor’s response to capsaicin in cells. The active ingredient in pepper is capsaicin.
When the mice were given capsaicin and fed peppery chicken feed, the researchers found no evidence of pain avoidance behavior, even in those with the K710N mutation. This was in contrast to normal mouse behavior, which was to lift their paws as a reflex to avoid contact with capsaicin, which was thought to cause pain upon skin contact.
The mice with the K710N mutation were less sensitive to nerve injury, but their response to painful heat was unchanged. Furthermore, when the K710 area in regular mice was blocked, their immediate behavioral response to painful stimuli decreased, and their nerve injury-induced hypersensitivity returned to normal levels.
TRPV1 not only regulates pain but also protects against various other stimuli. According to recent research, it functions as an internal molecular detector in non-neural cells, protecting them from cellular stress caused by glucose or tissue ischemia. Furthermore, despite the mutation, experiments involving heart muscle cells exposed to hydrogen peroxide, high glucose levels, and cerebral ischemia confirmed the shielding effect.
The importance of translational analysis
In the next phase of the research, the scientists aimed to decrease the receptor’s activity pharmacologically. They accomplished this by designing a peptide called V1-cal, which targeted the K710 area exclusively. When administered to mice along with capsaicin, V1-cal demonstrated a reduction in nociceptive responses and a decrease in the release of neuropeptides, which can lead to inflammation and swelling in the nerves without affecting the temperature. Additionally, the mice’s chronic pain showed a notable improvement.
Conclusion
The scientists hope to increase the significance of this study by verifying the results in laboratory settings that meet the highest standards mandated by regulatory organizations. Furthermore, they intend to investigate additional compounds that can be synthesized more easily than the peptide, conduct preliminary assessments, and proceed to a clinical trial if the preclinical trials yield positive results.
Article Source: Reference Paper | Reference Article
Learn More: