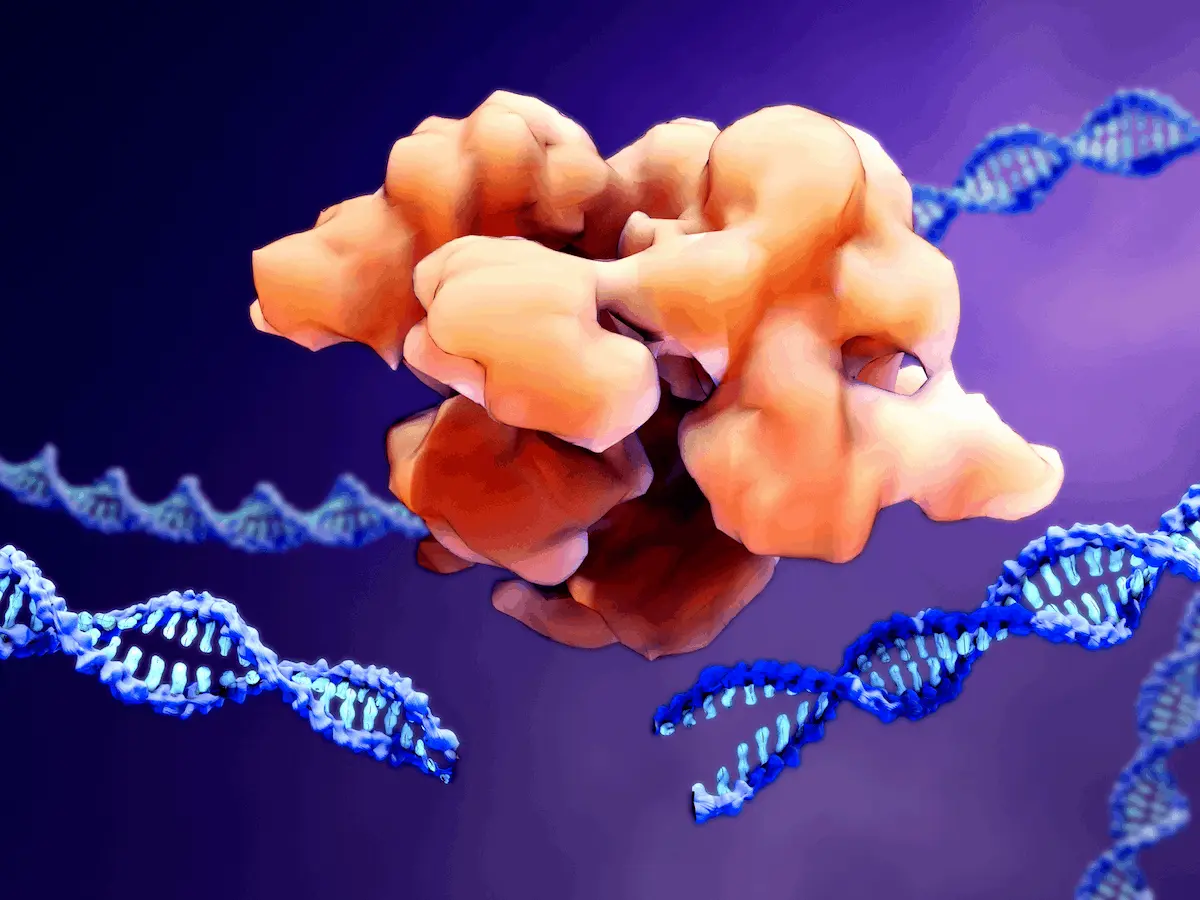
Through the advent of CRISPR-Cas12, scientists have managed to unlock a new world of gene editing and reprogramming, with important applications for diagnostics, therapeutics, agricultural science, and more. However, their bacterial origin may make them difficult to work with as researchers begin to explore the potential of human genome editing. A new study led by researchers from MIT found thousands of new RNA-guided enzymes known as Fanzors prevalent among eukaryotic organisms that show great promise as novel tools for mammalian genome editing.
RNA-programmable DNA nucleases are essential for the function of prokaryotes in the defense and proliferation of mobile elements. Such nucleases include the likes of CRISPR argonauts, as well as the nucleases that comprise the OMEGA systems (Obligate Mobile Element Guided Activity), which includes nucleases such as IscB, IsrB, and IshB, among others. Of these, one nuclease, the TnpB gene, contains a nuclease domain similar to the one found in the Cas12 gene, suggesting an evolutionary relationship between the two. This is further backed up by phylogenetic analysis conducted on the two domains, which indicates that different subtypes of Cas12 originated independently from different groups of TnpB enzymes. Furthermore, biochemical and cellular experiments have demonstrated that the TnpB-Omegas complex is an RNA-guided and programmable DNA endonuclease.
Proteins containing RuvC are not confined solely to prokaryotic organisms. A family of homologous proteins, known as Fanzors, has also been identified within eukaryotic organisms. These Fanzors exhibit a level of diversity comparable to that of their prokaryotic counterpart, TnpBs, and have been detected across various eukaryotic lineages, encompassing fungi, metazoans, amorphea, algae, as well as certain viruses. Notably, the identified Fanzors can be categorized into two primary groups: firstly, the Fanzor1 proteins, which are linked to eukaryotic transposons such as Mariners, IS4-like elements, Sola, MuDr, and Helitron and are predominantly prevalent within diverse eukaryotes. Secondly, the Fanzor2 proteins are affiliated with IS607-like transposons and are found in the genomes of dsDNA viruses. Despite the apparent similarities that exist among Fanzors and TnpB, the latter have not undergone a comprehensive survey and have remained largely uncharacterized experimentally.
An extensive survey of RNA-guided nucleases in eukaryotic and viral genomes has now been carried out, revealing a broad category of nucleases that have become widespread in both eukaryotic organisms as well as their associated viruses. Hence, the diversity of Fanzor systems was investigated within eukaryotes, and phylogenetic analysis was performed such that their evolution could be traced. Their programmability was demonstrated, with Fanzors showing promise as novel gene editing mechanisms.
RNA-guided nucleases were identified from more than 22000 eukaryotic as well as viral assemblies gathered from NCBI’s GenBank. More than 3000 such nucleases were found, all with unique gene sequences, which were found across various classes of eukaryotes. Fanzor homologs often were shown to have multiple copies inside genomes, suggesting strong intragenomic mobility. In addition, these proteins were noted to be significantly larger than TnpBs. Distinct clades of Fanzor nucleases were revealed through phylogenetic analysis, along with more systems that couldn’t be assigned to any one clade on the basis of phylogenetic analysis. These proteins consist of multiple domains and the RuvC-esque domain it shares with the Cas12 protein family.
It was also found that there were common associations between transposons and Fanzors, with the most frequent one occurring with the hAT transposon, suggesting an important role they play in association with these transposons. The clades 1a, 1b, and 1d are commonly shown as linked to hAT, while the 1c clade is instead related to CMC, LINE, and Mariner transposons. The Fanzor2 clade was also associated with an array of different transposons, including IS607, which has a transposase similar to TnpA, cementing its similarity with TnpB.
Fanzors are primarily associated with structured and conservative non-coding RNAs – when a Fanzor2 nuclease was selected from A. polyphaga, it was noted that strong conservation was shown in both protein-coding as well as non-coding regions. In-silico analysis showed that a stable folding was likely and that the region’s transcript could feasibly be a guide RNA associated with Fanzors, which were later named Fanzor RNA or fRNA. It was hypothesized that it, along with ApmFNuc, formed a complex that directed the binding and cleavage of DNA to a target-specific site. The aforementioned Fanzor sequence was expressed in an E. coli chassis. The ApmFNuc protein was notably unstable and required the presence of fRNA for stability. This is similar to TnpB’s instability when not in the presence of ωRNA. When purified and sequenced, enriched coverage was revealed between the protein’s IRR and ORF at the 3’ ends, showing evolutionary conservation.
The locations where RNA-guided nucleases performed cleavage varied significantly, and the patterns were mapped using Sanger sequencing. At the 3’ end, it was noticed that its cleavage patterns were quite similar to those exhibited by Cas12 and TnpB and the properties shown by programmable RuvC-like domains. The relative preference for different sites was quantified through the use of an assay based on NGS, revealing a cleavage pattern that was slightly different from that exhibited by TnpB nucleases. It was also demonstrated that these nucleases contained conserved catalytic sites and lacked collateral activity. They also contained nuclear localization signals (also known as NLS), which allowed them to cross the membrane of the nucleus. The nucleases were then tested for viability in mammalian genome editing, with experimental testing showing that nucleases from both Fanzor2 and Fanzor1 could be used for genome editing in humans.
Conclusion
The potential of Fanzor nucleases for effective genome editing was successfully demonstrated in human cells, showcasing their ability to induce cleavage and generate indels with measurable activity. These Fanzor nucleases, characterized by their compact size of approximately 600 amino acids, hold promise for enhanced delivery, while their eukaryotic origins may contribute to reducing their immunogenicity when applied in human systems. However, it is worth noting that further engineering efforts are necessary to enhance the performance of these nucleases in human cells, a development trajectory similar to that seen with other compact RNA-guided nucleases like the Cas12f protein often used in CRISPR applications.
Additionally, the presence of Fanzor nucleases across multiple eukaryotic lineages and their associated viruses strongly suggests that numerous undiscovered RNA-guided systems within the realm of eukaryotes still exist. This could be an invaluable tool for future exploration, characterization, and the development of innovative biotechnological applications.
Article source: Reference Paper
Learn More:
Sonal Keni is a consulting scientific writing intern at CBIRT. She is pursuing a BTech in Biotechnology from the Manipal Institute of Technology. Her academic journey has been driven by a profound fascination for the intricate world of biology, and she is particularly drawn to computational biology and oncology. She also enjoys reading and painting in her free time.













[…] MIT Scientists Discover Thousands of New Programmable DNA-Cutting Enzymes Opening the Door to New To… […]