To fight cancer, we must stop their metastasis or uncontrolled multiplication. Hence, it is important to understand what proteins these cancer cells need for growth. Precision medicine is supported by our understanding of the genetic makeup of humans, but the genotype-phenotype relationships remain therapeutically unactionable because of less functional assays and tools. In the rapidly developing field of chemical biology, the building and utilization of sophisticated approaches to probing protein interactions are central to extending the knowledge of cellular processes and therapeutic development. Researchers from the Department of Chemistry, Scripps Research, CA, Vividion Therapeutics, San Diego, CA, Chemical Biology and Therapeutics Science Program, Broad Institute, Cambridge, and the Department of Chemistry and Chemical Biology, Harvard University, present a new paradigm in the exploration of the human proteome through the application of covalent chemistry in combination with Activity-Based Protein Profiling. This study yields fresh insights into protein-electrophilic small molecule interactions that have great bearings on cancer research and drug development. Identifying the right cancer target is essential for these advancements.
Covalent Chemistry and Activity-Based Protein Profiling
Covalent chemistry has so far provided us with an effective strategy for expanding the ligandability of proteins, particularly toward targets with shallow or otherwise intractable binding sites. Approaches typically rely on electrophilic small molecules that form covalent adducts with nucleophilic amino acid side chains in proteins. Improved selectivity and a prolongation of pharmacological activity are usually added to such covalent approaches; a very important property to be pursued in the case of certain proteins that are not targetable by classic drug discovery approaches.
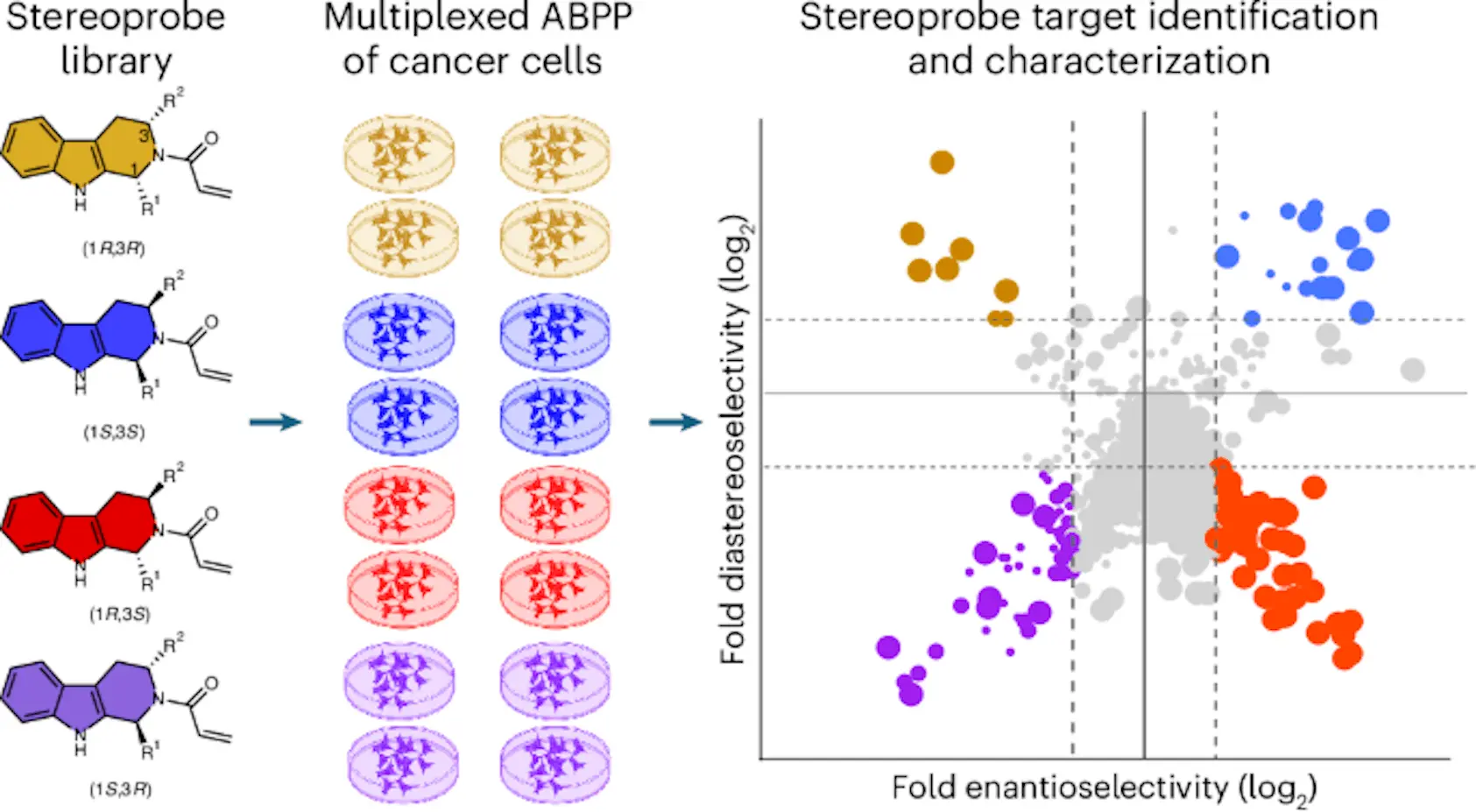
Accordingly, ABPP (Activity-Based Protein Profiling) has emerged as a quite versatile technology. By competing with broadly reactive probes (tools or substances used to detect, measure, or interact with specific biological molecules in a system), electrophilic compounds are able to determine ABPP, which modifies nucleophilic residues in proteins, thus giving a direct readout of protein-small molecule interactions. Electrophilic compounds have a dearth of electrons, while nucleophilic compounds have an excess of them. The researchers used the described approach to study the stereochemical interactions of the proteins of cancerous cells with a stereochemically defined electrophilic set of compounds, the tryptoline acrylamide stereoprobes.
Design and Application of Tryptoline Acrylamide Stereoprobes
To chart protein interactions in cancer cells, the researchers prepared five sets of alkyne-functionalized tryptoline acrylamide stereoprobes, each comprising four stereoisomers (same number of atoms, but different chemical properties). These probes were evaluated for their reactivity using gel-based ABPP and MS (mass spectrometry) analysis on two human cancer cell lines, 22Rv1 (prostate carcinoma) and Ramos (B lymphoid cells), with distinct gene expression profiles, thus offering complementary portraits of protein ligandability.
Initial experiments now showed that trans (1R,3S) and the (1S,3R) stereoprobes were much more reactive than their respective cis-stereoisomers. This stereoselective reactivity was further confirmed in protein-directed ABPP experiments using a panel of enzymes wherein proteins enriched more than threefold by one stereoprobe over its enantiomer were considered stereoselective targets. More importantly, it stereoselectively enriched more than 150 proteins at low concentrations such as 5 µM with higher enrichment ratios compared with higher concentrations such as 20 µM.
Integration of Protein- and Cysteine-Directed ABPP
These researchers obtained the comprehensive map of protein interactions through the merging of data from protein-directed and cysteine-directed ABPP experiments. By the cysteine-directed approach, the authors were able to detect the cysteines covalently modified by the stereoprobes with the iodoacetamide probes. From over 9000 proteins, more than 38,000 cysteines were identified, where 238 cysteines on 217 proteins were stereoselectively liganded.
This integration identified 336 stereoselectively liganded proteins across an impressive breadth of functional classes, including enzymes, channels/transporters, transcription/translation factors, and adaptor/scaffolding proteins. Notably, 40% of such hits resulted in proteins that were marked as essential upon examination of the Cancer Dependency Map, thereby justifying their potential as targets of interest.
Insights & Results from Protein and Cysteine-Directed Data
Discussion of one of the most important observations of this work relates to the fact that some proteins were stereoselectively liganded by one or both ABPP platforms, while others were identified only by one approach. For instance, larger proteins like HECTD4 and PRKDC, which had a number of cysteines liganded by distinct stereoprobes, were more likely to be identified by cysteine-directed ABPP. This would imply that protein size and the presence of multiple ligandable cysteines might complicate interpretation by protein-directed activity-based probes.
Conversely, certain proteins were detected only by means of protein-directed ABPP because their interactions relied on the alkyne modification per se. For example, the RNA-binding proteins STRBP and FXR1 were stereoselectively enriched using the stereoprobes in the absence of corresponding cysteine modifications, suggesting that the protein-directed approach can capture ligandable interactions that are not dependent on cysteine.
They reported that AFMID is one of the important enzymes in the tryptophan-kynurenine metabolic pathway and is stereoselectively liganded by the stereoprobe WX-01-03 (1R,3R configuration). The interaction decreases hydrolytic conversion of N-formylkynurenine to kynurenine and may modulate immune responses.
The other major finding was the determination of an adaptor protein, MAD2L1BP, as a target for the stereoprobe WX-03-341 in the SAC complex. This interaction had been demonstrated to abrogate MAD2L1BP from binding to the SAC complex, thereby delaying mitotic exit in cancer cells! This finding underlines the potential of stereoprobes in investigating and disrupting protein-protein interactions critical to cell division and cancer progression.
Conclusion
The multitiered chemical proteomic approach described herein represents the big leap forward in our capabilities to map protein interactions in cells. The authors combined information from protein- and cysteine-directed ABPP data and created a near-global snapshot of the ligandability of the human proteome in cancer cells. Stereoselective liganding events, identified in such a wide array of proteins, open new pathways not only in drug discovery but also in the development of targeted therapies. Studies such as this are going to be very important in continuing to shape the future of precision medicine and cancer treatment as proteomics continues to evolve!
Article Source: Reference Paper Abstract | Reference Article | GitHub.
Follow Us!
Learn More:
Neermita Bhattacharya is a consulting Scientific Content Writing Intern at CBIRT. She is pursuing B.Tech in computer science from IIT Jodhpur. She has a niche interest in the amalgamation of biological concepts and computer science and wishes to pursue higher studies in related fields. She has quite a bunch of hobbies- swimming, dancing ballet, playing the violin, guitar, ukulele, singing, drawing and painting, reading novels, playing indie videogames and writing short stories. She is excited to delve deeper into the fields of bioinformatics, genetics and computational biology and possibly help the world through research!