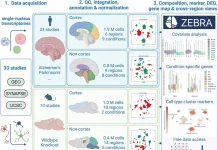
Scientists from Brown University identified the novel usage of FDA-approved drugs by cluster analysis of behavioral profiles for the treatment of neurodegenerative disorders by utilizing CsA-type drugs, which are known to impact the neural system. The study suggests that drugs with ‘CsA-type’ behavioral profiles are potential candidates for preventing and treating Alzheimer’s disease.
Image Source: Novel use of FDA-approved drugs identified by cluster analysis of behavioral profiles
Repurposing FDA-approved drugs is a productive, efficient, and cost-effective approach to the development of therapeutics for an expansive scope of diseases. Nonetheless, prediction of function can be challenging, particularly in the brain.
The scientists screened a small molecule library with FDA-approved drugs for consequences on behavior. The studies were completed utilizing zebrafish larvae, imaged in a 384-well format.
The scientists observed that different drugs influence activity, habituation, startle responses, excitability, and optomotor responses. The progressions in behavior were organized in behavioral profiles, which were inspected by hierarchical cluster analysis.
One of the distinguished clusters included the calcineurin inhibitors cyclosporine (CsA) and tacrolimus (FK506), which are immunosuppressants and likely therapeutics in the prevention of Alzheimer’s disease.
The calcineurin inhibitors structure a functional cluster with apparently unrelated drugs, including bromocriptine, tetrabenazine, rosiglitazone, nebivolol, sorafenib, cabozantinib, tamoxifen, meclizine, and salmeterol.
The scientists proposed that drugs with ‘CsA-type’ behavioral profiles are promising possibilities for avoiding and treating Alzheimer’s disease.
Repurposing Drugs
Repurposing drugs has been proposed to treat different diseases, including cancer, neurodegenerative disease, and Alzheimer’s disease.
Repurposing is practical, effective, and cost-efficient since existing drugs have previously passed clinical preliminaries to assess safety.
New use might be found for drugs that have been approved by the U.S. Food and Drug Administration (FDA), as well as the failed drugs that passed stage I clinical preliminaries to assess safety yet were not viable in stage II or III clinical preliminaries.
FDA-approved drugs with expired licenses might also be viable, and new users can be assessed in population studies before starting extra clinical preliminaries.
Drug repurposing is an especially strong methodology for small molecule treatment of neural disorders. Small molecules are bound to pass the blood-brain barrier than protein-based biologics, which are typically excluded from the brain.
Likewise, numerous molecular targets situated in visceral systems are additionally present in the brain. For instance, angiotensin receptors in blood vessels are prime targets for blood pressure medicine. These receptors are likewise found in neurons, astrocytes, oligodendrocytes, and microglia in the brain, demonstrating that angiotensin inhibitors might be reused for the treatment of neural disorders.
History of Drug Repurposing
There is a rich history of fruitful drug repurposing, which incorporates classical models, for example, acetylsalicylic acid (Aspirin) and sildenafil (Viagra).
Acetylsalicylic acid was first marketed by Bayer in 1899 for help in pain relief and was reused during the 1980s to prevent heart attacks.
Sildenafil was studied in 1985 by Pfizer for the treatment of hypertension yet was reused and promoted in 1998 for erectile dysfunction. In 2005, it was reused again for pulmonary hypertension.
The Alzheimer’s Disease
Alzheimer’s disease has been a challenging disorder for drug development, with numerous competitor drugs failing in clinical trials. These difficulties might be caused to some degree by determining molecular targets that assume a part in late neurodegenerative processes instead of the early signaling pathways that cause Alzheimer’s disease.
One of the early signaling proteins that are thought to assume a key part in Alzheimer’s disease is the calcium-dependent serine-threonine phosphatase calcineurin.
The accompanying model has been proposed: Intracellular free calcium increases in the aging brain because of oxidative stress, mitochondrial dysfunction, and amyloid β oligomers that bind transmembrane proteins.
A subtle yet drawn-out increase in intracellular calcium activates the phosphatase calcineurin. Calcineurin eliminates the phosphate group of signaling proteins, like the nuclear factor of activated T-cells (NFAT), glycogen synthase kinase-3 (GSK-3), and BCL2-associated death protein (BAD). These signaling proteins then activate molecular and cellular processes related to Alzheimer’s disease.
In light of this model, calcineurin inhibitors are considered suitable therapeutics for the beginning phase of Alzheimer’s disease. This thought is upheld by population studies, which show that transplant patients treated with the calcineurin inhibitors cyclosporine A (CsA) or tacrolimus (FK506) rarely foster Alzheimer’s disease in all age groups over 65.
In transplant medication, CsA and FK506 are utilized as immunosuppressants to forestall organ rejection. However, small molecules like CsA and FK506 in their impact on the brain don’t suppress the immune system, which might be ideal candidates for the treatment of Alzheimer’s disease.
Nonetheless, function prediction is challenging for small molecules, particularly in the brain. Small molecules frequently have numerous molecular targets and can influence communicating signaling pathways in complex neural networks.
The analysis of behavior in animal model frameworks offers an answer since subtle changes in neural function can be recognized, in any event, when the brain appears to be unaffected by molecular or structural criteria.
The Current Study on Examination of Behavior in Zebrafish Larvae
In the current study, the scientists inspect behavior in zebrafish hatchlings. Zebrafish are a well-known model framework in the biomedical sciences and are an incredibly encouraging model for human brain disorders.
Zebrafish larvae can be imaged in vivo in microplates, and explicit ways of behaving can be estimated via automated image analysis. Besides, high-throughput investigations of behavior have been utilized to screen small molecule libraries, which prompted the discovery of novel drugs with clinical relevance.
The scientists treated larvae with FDA-approved drugs and estimated a wide scope of behaviors, activity, habituation, acoustic startle responses, excitability, and optomotor responses. These ways of behaving in zebrafish are not straightforwardly linked to the behaviors of individuals with Alzheimer’s disease.
Be that as it may, zebrafish larval behaviors are controlled by a wide range of neural signaling pathways, including calcineurin signaling pathways. Drugs that focus on these pathways will influence explicit parameters of zebrafish behavior and might be utilized to target comparative signaling pathways in human brain disorders.
The zebrafish behaviors were then summed up in behavioral profiles, which were analyzed for similarity by hierarchical cluster analysis. This cluster analysis uncovered various unrelated drugs with ‘CsA-type’ behavioral profiles.
The Endpoint
The current study shows that a wide range of FDA-approved drugs influence behavior. Changes in behavior were summed up in behavioral profiles, which were analyzed by hierarchical cluster analysis.
The cluster analysis was done utilizing 190 FDA-approved drugs as well as small molecules that influence the calcineurin-NFAT signaling pathway. This signaling pathway has been portrayed in detail in the immune system during T-cell activation yet may likewise assume a critical part in the regulation of neural function and behavior.
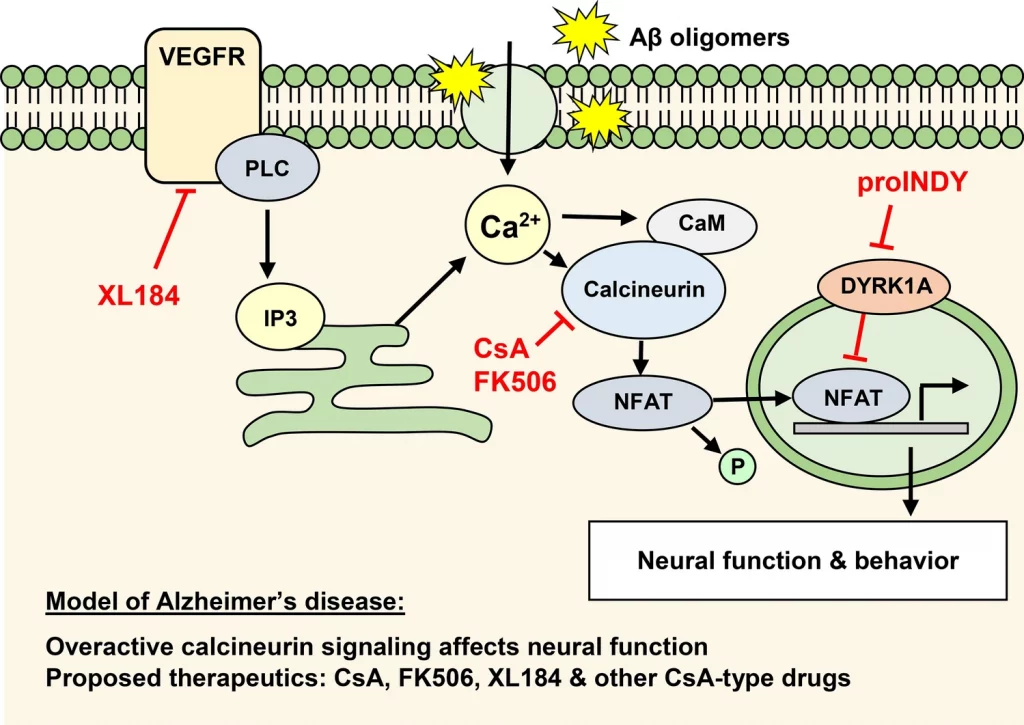
Image Source: Novel use of FDA-approved drugs identified by cluster analysis of behavioral profiles
The cluster analysis distinguished a group of 11 FDA-approved drugs with CsA-type impacts on neural function. Two of these drugs, sulfasalazine, and irbesartan, didn’t induce a CsA-type profile in the validation experiments and were eliminated from the scientists’ rundown of CsA-type drugs.
The accompanying 9 CsA-type drugs were validated in the study: bromocriptine, tetrabenazine, rosiglitazone, nebivolol, sorafenib, cabozantinib, tamoxifen, meclizine, and salmeterol.
Article Source: Tucker Edmister, S., Del Rosario Hernández, T., Ibrahim, R. et al. Novel use of FDA-approved drugs identified by cluster analysis of behavioral profiles. Sci Rep 12, 6120 (2022). https://doi.org/10.1038/s41598-022-10133-y
Tanveen Kaur is a consulting intern at CBIRT, currently, she's pursuing post-graduation in Biotechnology from Shoolini University, Himachal Pradesh. Her interests primarily lay in researching the new advancements in the world of biotechnology and bioinformatics, having a dream of being one of the best researchers.