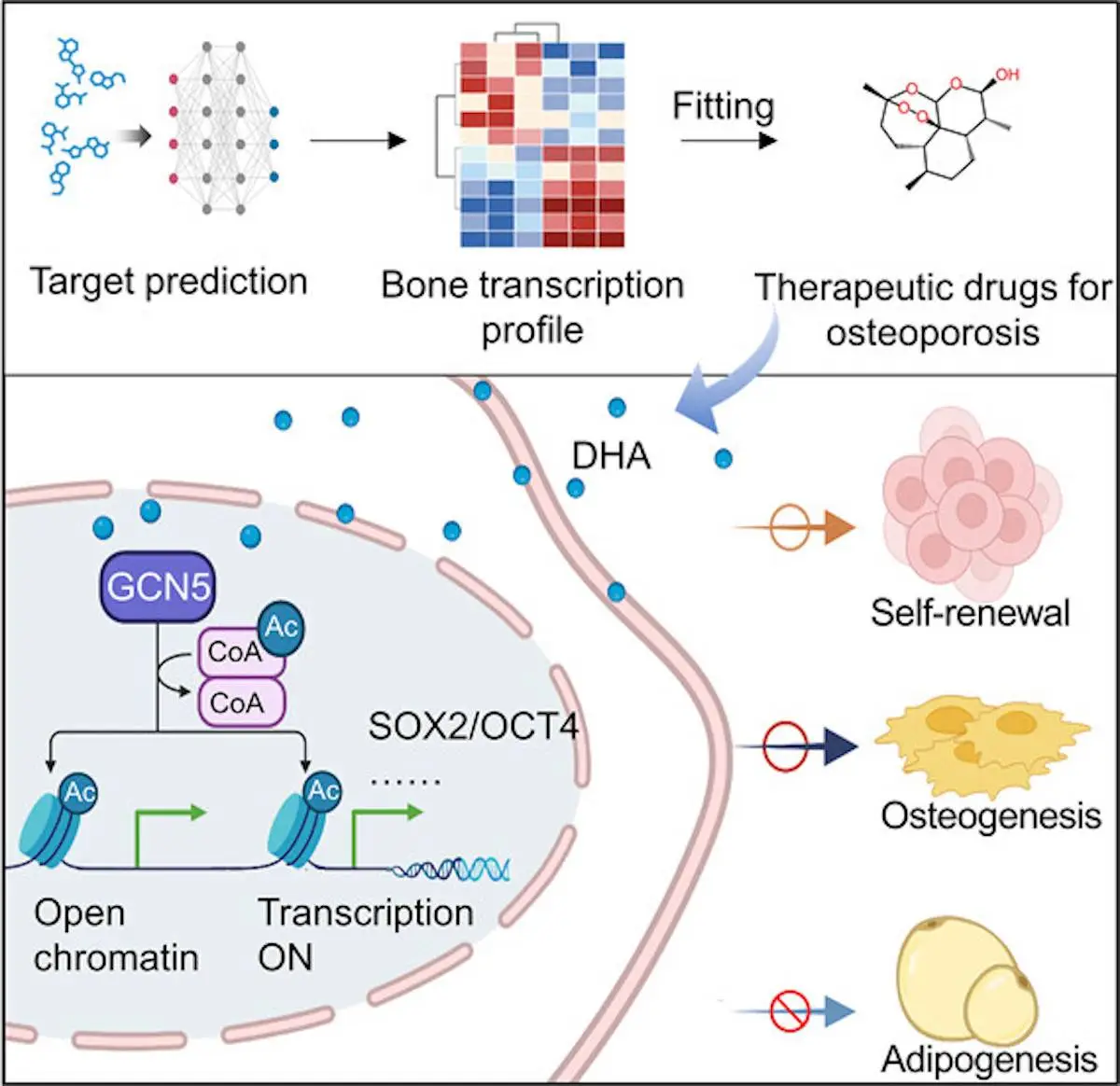
Scientists at Peking University School and Hospital for Stomatology, China, have used deep learning to identify dihydroartemisinin (DHA), an antimalarial drug, as a potential treatment for osteoporosis. DHA was found to maintain the stemness of mesenchymal stem cells (MSCs), which are important for bone formation. In mice, DHA treatment was able to reverse osteoporosis-related bone loss. The research published in ACS Central Science suggests that DHA could be a promising new therapeutic agent for osteoporosis.
Osteoporosis is a disease that affects millions of people worldwide. Bone density is significantly decreased, and the microstructure of the bone is degraded slowly. There is a disruption in the delicate balancing act between the formation and degeneration of bone due to the malfunctioning of osteoblasts. The precursors of these osteoblasts, Bone marrow-derived mesenchymal stem cells or BMMSCs, have important roles in osteoporosis. These stem cells steadily proliferate and differentiate into functional osteoblasts, allowing for bone density to be maintained. However, in patients with osteoporosis, these stem cells display a tendency to differentiate into adipocytes instead and have reduced regenerative potential. The current treatments for osteoporosis primarily target the remediation of deficiencies in hormone production as well as bone resorption but cannot target the root cause: the lost stemness and regenerative capacity of the precursor stem cells. However, new deep learning tools can help remedy this shortcoming by targeting the problem at its source.
Many approaches have been designed in order to restore the capacity of these stem cells, including chemical and physical stimulation, gene editing, medication, and cytokines, among others. In recent years, computational methods involving deep learning have increasingly become a mainstay in the biotechnology field, allowing researchers to accelerate the timeline of research and reduce development and operational costs while simultaneously being able to analyze vast amounts of data. Machine learning algorithms can accurately predict the efficacy of certain drugs as well as responses to treatment and have already been utilized successfully in the development of drugs for various diseases such as obesity and hyperuricemia.
The deep learning-based efficacy prediction system (also known as DLEPS) was utilized to compute efficacy scores depending on genes that were expressed in bone tissue from neonatal mice when compared against those of adult mice. Among these, dihydroartemisinin, a common treatment found in traditional Chinese medicine, was identified as a potential candidate for the promotion of bone homeostasis as well as BMMSC stemness. In vitro analysis confirmed these characteristics, and the administration of DHA to the bone tissues was shown to inhibit density loss while maintaining BMMSC functionality. DHA was found to upregulate genes controlling stemness to allow for their downstream expression.
Differentially expressed genes in femur tissue were identified in neonatal and adult mice through the use of RNA sequencing, as stemness was noted to decrease as aging progressed. DLEPS was then used to predict small molecules, which could be used to reverse the blocking of expression in these genes, allowing for an overall increase in stemness and functionality. In order to determine the efficacy of DHA, a replicative cellular senescence model was used. DHA’s efficacy against differentiation was also evaluated by comparing DHA-treated stem cells and a control group, which proved that the former retained their stemness and ability to proliferate and regenerate for far longer than stem cells that weren’t treated with DHA. It was then found that the delivery of DHA through intragastric administration significantly reduced the effects of osteoporosis in mice when induced by ovariectomy. The presence of DHA allows for mouse BMMSCs to maintain stemness as well as unbiased differentiation potential.
It was also found that mesenchymal stem cell regulation is majorly influenced by epigenetic regulation. The structure and activity of these genes undergo dynamic changes when going through various biological processes, such as chromatin remodeling and histone modifications. It was previously verified that lowered transcriptional activity due to the presence of hypoacetylated histone lysines had been observed in human BMMSCs before. The levels of histone deacetylase, as well as histone acetyltransferase, differed in osteoporotic and on-osteoporotic mice, proving the existence of a correlation between them and the progression of the disease.
From this, it was hypothesized that the presence of DHA may result in the epigenetic regulation of the transcriptional activity of enzymes controlling acetylation, hence controlling the fate of the stem cells. This was verified through the use of in vitro testing and analysis of cells with and without exposure to DHA. It was thus proven that DHA helps maintain the stemness of BMMSCs through upregulating acetylation of Histone 3 Lys 9. Mesoporous silica nanoparticles and bone-targeting alendronate were conjugated to form nanospheres with which the DHA could be delivered. Alendronate is traditionally used as an anti-osteoporosis drug, and it has a high affinity to the surface of the bone. The MSN-ALN conjugates were biodegradable in the conditions found in the microenvironment where they were to be used.
Conclusion
This novel therapy for osteoporosis could be instrumental in changing the lives of the millions of people who suffer from this disease worldwide. Osteoporosis is common among older people and often leads to a reduced quality of life. It also results in greater severity and occurrence of bone fractures, which may lead to chronic pain as well as reduced ability to perform physically demanding activities. Due to a lack of symptoms until the occurrence of a fracture, it is excessively tough to medicate osteoporosis effectively. This difficulty may be alleviated with the introduction of DHA as a potential therapeutic tool. The use of deep learning models to identify the usefulness of DHA in treating osteoporosis serves as further proof of the usefulness and efficacy of computational methods in biological and medical research. By reducing the time required to collect and analyze data thoroughly, it was possible to quickly and efficiently produce a potential cure. Such methods can be used for other diseases as well to find other candidates that can be useful for therapeutic interventions. When combined with in vitro analysis for testing and validation, the drug discovery and development process can be significantly shortened.
Article source: Reference Paper
Learn More:
Sonal Keni is a consulting scientific writing intern at CBIRT. She is pursuing a BTech in Biotechnology from the Manipal Institute of Technology. Her academic journey has been driven by a profound fascination for the intricate world of biology, and she is particularly drawn to computational biology and oncology. She also enjoys reading and painting in her free time.