The development of new mechanisms for antibiotics is essential to combating antimicrobial resistance. With its unique chemical scaffold, Teixobactin is a new class of antibiotics that lacks detectable resistance. A precursor of peptidoglycan, lipid II, is targeted by Teixobactin. Teixobactin binds specifically to the sugar moiety of lipid II at its C-terminal enduracididine headgroup, whereas another lipid II molecule coordinates its N-terminal pyrophosphate. In order to destroy the membrane, Teixobactin hijacks lipid II. Antibiotics that act on membranes also damage human cells, causing unwanted side effects. A lipid II deficiency in eukaryotes explains why Teixobactin damages only those membranes that contain lipid II. Efficacious compounds target bacterial cell envelopes with a double-pronged approach that targets cell wall synthesis and the cytoplasmic membrane. To develop improved drug candidates, a structural understanding of teixobactin is essential.
Antibiotic resistance is a major concern for global health due to the rapid increase in multidrug-resistant bacteria. Recent decades have seen few new classes of antibiotics introduced into the clinic, exacerbated by a drought in the antibiotic pipeline. The formation of teixobactin-lipid II fibrillar structures results in membrane defects, which contribute to the killing mechanism of teixobactin, demonstrated by ssNMR, HS-AFM, confocal microscopy, and computer simulations. Teixobactin kills bacteria more effectively than vancomycin, which binds to the pentapeptide of lipid II by attacking the membrane.
The production of membrane-acting antibiotics that have no adverse effects on human cells has so far proved challenging. As a result of its selectivity, teixobactin disrupts membranes only when supramolecular structures are formed and only if the membrane carries lipid II. In contrast to conventional one ligand-one target paradigms, teixobactin forms a supramolecular structure. Teixobactin’s ability to inhibit peptidoglycan synthesis as well as disrupt the membrane provides an effective means for attacking the bacterial cell envelope, whereas binding to immutable target results in an antibiotic that cannot be resisted.
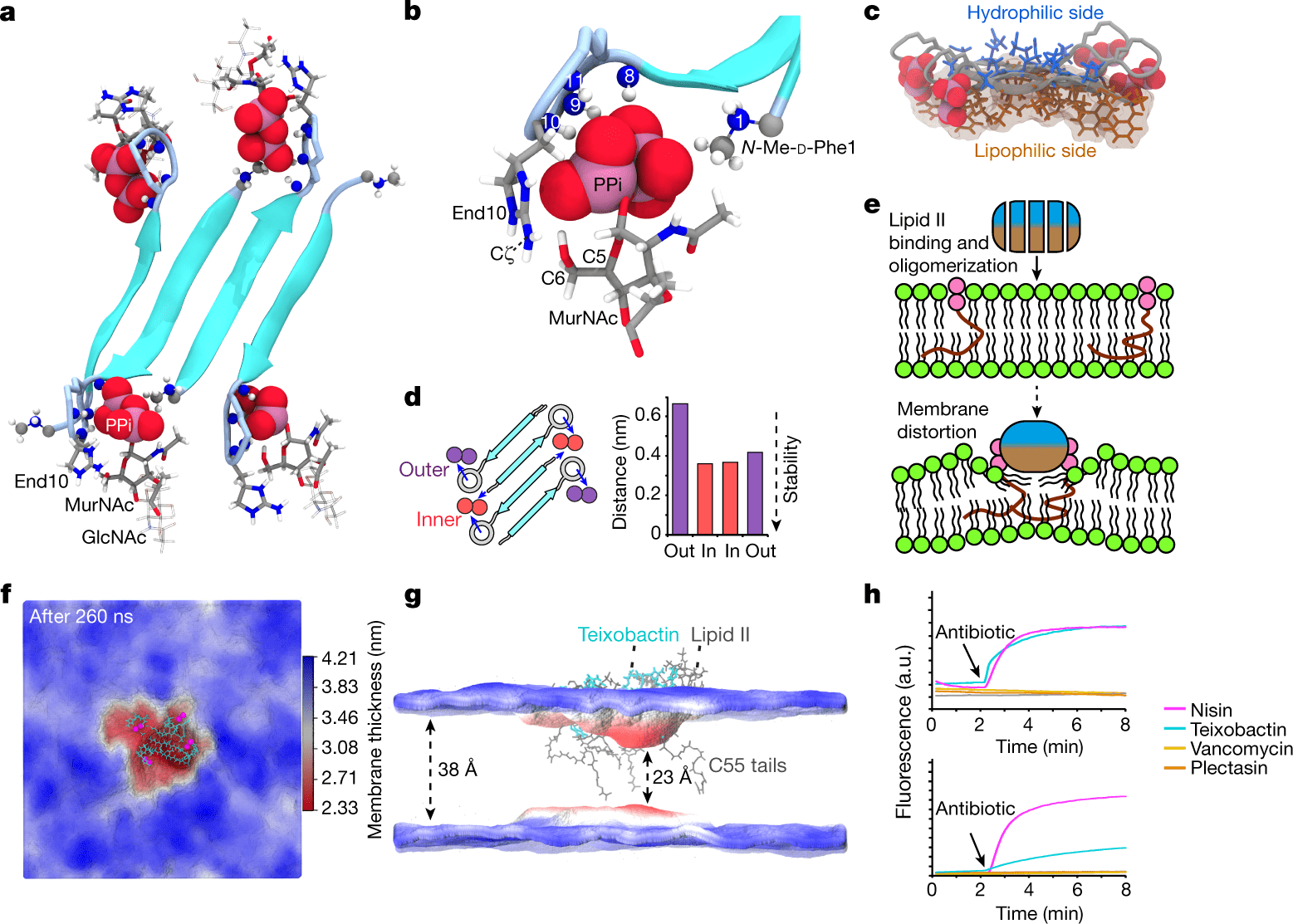
Teixobactin exists as an undecapeptide containing five non-canonical amino acids, including four d-amino acids and the cationic l-allo-enduracididine (End10), located in a depsicycle at its C-terminus. There is considerable interest in enduracididine because its five-membered cyclic guanidinium moiety is rarely found in nature and is prominently displayed at the putative lipid II binding site. Teixobactin or its analogs have been synthesized in complex ways by several groups, but the role of End10 in the mechanism of action remains unclear.
Intermittent localization of l-amino and d-amino acids permits the formation of an antiparallel beta-sheet interaction between two adjacent teixobactin molecules. A PPi-coordinating sequence in the N terminus of teixobactin contributes to target capture. Upon binding to lipid II, teixobactin forms a supramolecular fibrillar structure. These structures are likely irreversible, acting as sinks that concentrate teixobactin at the site of action. The supramolecular structure not only sequesters lipid II efficiently and effectively but also displaces phospholipids, causing a thinned membrane and compromised integrity. In isolated molecules, teixobactin’s hydrophobic residues do not cause any damage due to their short length. This produces a concentrated hydrophobic patch when combined in a supramolecular structure.
Membrane damage caused by oligomerization
ssNMR spectra produced by liposomes contain a well-defined complex of teixobactin and lipid II. The addition of teixobactin to pyrene-tagged lipid II results in the formation of oligomers immediately. For Teixobactin to be effective, oligomerization is required upon target binding. N-terminally truncated constructs have significantly reduced activity. The effects of Teixobactin can last for a long time after the drug is administered, making it an effective treatment for bacteria that are slow to grow.
Using a complex interface
The glycopeptide antibiotic vancomycin is resistant to lipid II since it contains a conserved sugar-pyrophosphate (GlcNAc–MurNAc–PPi) part and a pentapeptide with a variation. Due to its inability to bind the pentapeptide of lipid II, teixobactin is less sensitive to pentapeptide composition variations than vancomycin. As a result, teixobactin is able to prevent the development of resistance. End10 and MurNAc interact tightly, constraining the pentapeptide’s flexibility. As a result, the pentapeptide has greater rigidity when combined with teixobactin than when combined with R4L10-teixobactin. Teixobactin might prevent transglycosylases of the cell wall biosynthesis from recognizing lipid II due to its obstruction of the pentapeptide’s conformational space.
Image source: https://doi.org/10.1038/s41586-022-05019-y
Complex structure
Teixobactin is well integrated with the PPi-MurNAc group of lipid II. Teixobactin depsi-cycle protons and the cationic N terminus of the neighboring strand of teixobactin tightly coordinate PPi in the oligomeric complex. The cationic N terminus of teixobactin improves the stability of the interface by dual coordination with lipid II. As a rule of thumb, natural teixobactin binds tightly to the invariant PPi–MurNAc part that exists in several cognate cell wall precursors, e.g., lipid I, lipid II, or lipid III (precursors to teichoic acid), while shunning the pentapeptide that is unique to each bacterial strain. Thus, natural teixobactin minimizes the likelihood of resistance development while maximizing its target spectrum.
R4L10-teixobactin has a fuzzy interface where the hydrophobic residues of the depsi-cycle do not preferably interact with MurNAc, which contrasts with the specific interface between natural teixobactin and PPi-MurNAc.
Membrane splayed by hydrophobic wedges
The perturbations must certainly be more pronounced in much larger oligomers of teixobactin–lipid II than in the simulated building blocks. Teixobactin’s antimicrobial activity may be attributed to the membrane perturbations caused by its supramolecular structure. A sharp decrease in membrane potential was observed when teixobactin was added to Gram-positive bacteria.
In the process of corrupting its target, teixobactin converts it into a membrane disruptor, joining a select group of other natural products that also cause target malfunctions, such as aminoglycosides that interrupt translation and acyldepsipeptide, which stimulates proteolysis. In addition to being membrane-active antibiotics, nisin has a large hydrophobic moiety that damages the membrane of bacteria as well as eukaryotic cells, causing toxicity.
Article Source: Shukla, R., Lavore, F., Maity, S., et al., Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature. (2022). https://doi.org/10.1038/s41586-022-05019-y
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Srishti Sharma is a consulting Scientific Content Writing Intern at CBIRT. She's currently pursuing M. Tech in Biotechnology from Jaypee Institute of Information Technology. Aspiring researcher, passionate and curious about exploring new scientific methods and scientific writing.