Scientists at the University of Toronto advanced drug formulation development by utilizing machine learning techniques to predict and guide the design of long-acting injectable drugs. Through the use of these algorithms, the scientists were able to predict the release of drugs from advanced delivery systems and use this information to inform the design of new long-acting injectables. This data-driven approach can potentially make the development process more efficient and cost-effective.
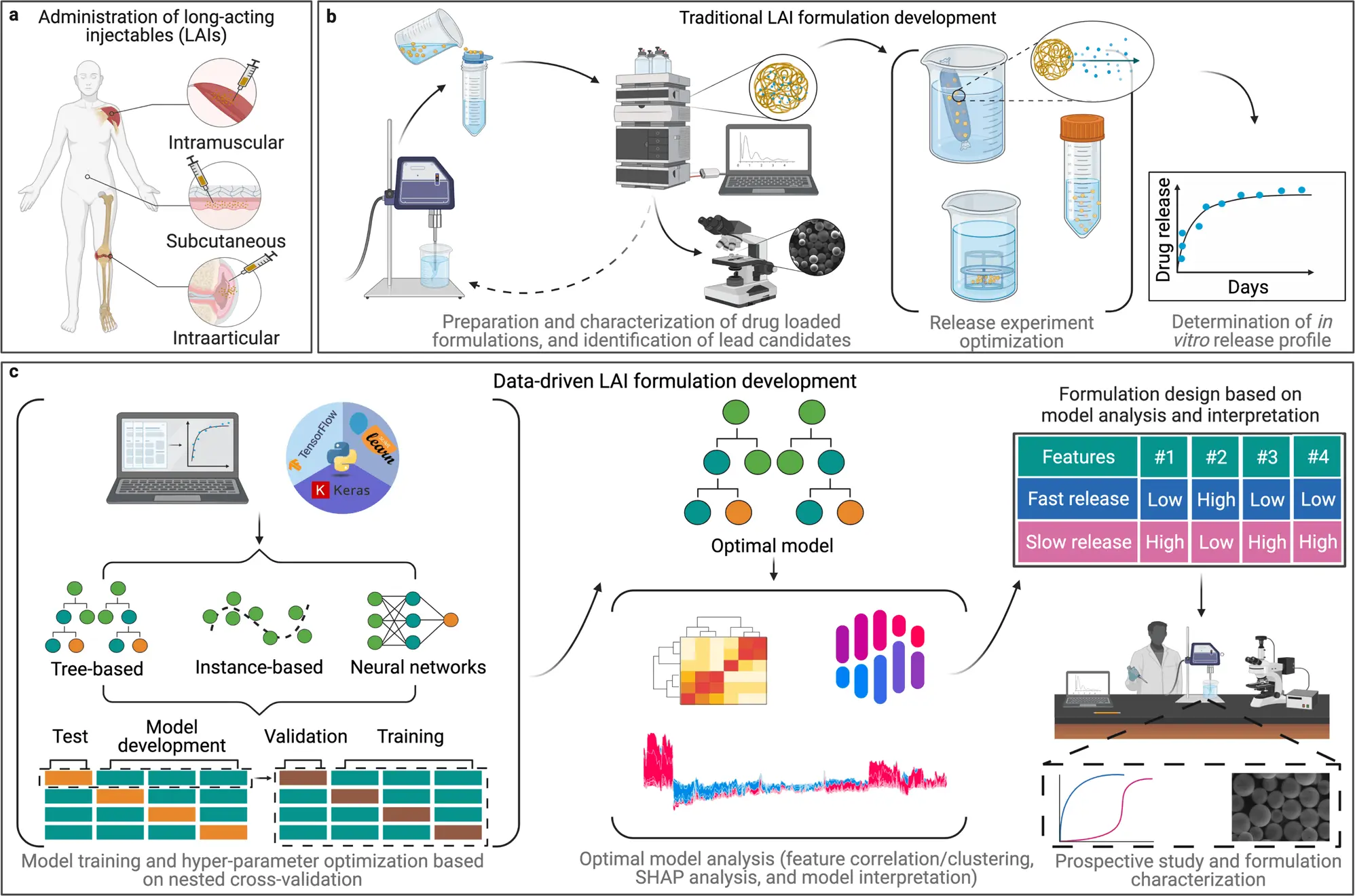
Long-acting injectable drugs, which can improve efficacy, safety, and patient compliance, are a promising strategy for treating chronic diseases. Polymer materials offer a wide range of properties, but predicting their performance in a drug formulation is difficult due to the interplay of multiple parameters. To overcome this challenge, extensive in vitro experimentation is often required. However, using machine learning models can accelerate the design of polymeric long-acting injectable drugs by enabling more efficient discovery and characterization of formulations. Machine learning has been making significant advancements in drug discovery and materials science.
Long-Acting Injectables (LAI)
Long-acting injectable drugs (LAIs) are specialized delivery systems that are used to release medication over a prolonged period of time to achieve a sustained therapeutic effect. These drugs are designed to be slowly released into the bloodstream over days, weeks, or even months, allowing for fewer injections and improved medication adherence.
These LAIs have several advantages over traditional drug formulations, such as improved patient adherence and increased drug bioavailability. LAIs can be engineered to deliver the drug to the body in different ways, locally or systemically, and can be used in the treatment of chronic diseases, making them a promising formulation approach.
Creating the perfect release of a drug over a specific period of time through long-acting injectable drugs is a complex process that requires a lot of trial and error in the form of extensive and time-consuming experiments to come up with the right combination and quantity of the ingredients used. This process of developing and testing a wide range of formulation candidates is quite labor-intensive and time-consuming, which is leading to a bottleneck in the development of long-acting injectable drugs compared to other types of drug formulations.
The current research is a significant advancement in data-driven drug formulation development, mainly focusing on long-acting injectable drugs. The author highlights that machine learning has dramatically contributed to the identification of potential new medicines by discovering new molecules. They aim to further expand upon this success by utilizing similar techniques to improve the design of drug formulations and therefore develop more effective medicines.
Utilizing Machine Learning to Streamline New Drug Development
The research team aimed to determine if machine learning algorithms could effectively predict the release rate of drugs. Eleven different models were trained and evaluated, including MLR, RF, lightGBM, and NN, using a dataset composed of previously published studies from the author’s work and that of other research groups.
The researchers divided the dataset into two subsets, one for training machine learning models and another for testing them. Afterward, the models were used to predict the results of the test subset, and the predictions were compared to previously collected experimental data. The research team found that the tree-based models, specifically the lightGBM model, produced the most accurate predictions.
Building on the predictions made by the machine learning models, the team then sought to demonstrate how these models could be used to inform the design of new LAI drugs. The researchers used advanced analytical techniques to extract key design criteria from the lightGBM model. Then they created a new LAI formulation for a drug used to treat ovarian cancer. By interpreting what the machine had learned and using that information to develop design criteria for new systems, the team could create a drug with the desired release profile in a single iteration rather than the multiple iterations that would have been required in the past. The drug release rate was then tested to validate the predictions made by the lightGBM model, which were consistent with the desired release rate.
The COVID-19 pandemic has shown increased urgency for the rapid development of new drug formulations. The emergence of new virus variants highlights the need to design and create new formulations quickly so that patients can have access to new treatments as soon as possible. The research team is exploring the use of machine learning to aid in the development of new formulations using mRNA and lipid nanoparticle technology. The ability to create new formulations quickly is vital to bring new treatments to patients.
Need to Address the Shortage of Open-Source Data in the Pharmaceutical Sciences
The study demonstrates the potential for machine learning to accelerate the development of long-acting injectable drugs. However, the researchers noted that a lack of open-source data sets in pharmaceutical sciences represents a significant challenge to future progress. There was a shortage of data reported in studies using polymeric microparticles, and this limited their ability to use the data to develop machine learning models to improve the development process. There is a need for robust databases in pharmaceutical sciences that are freely available to researchers so that they can work together to advance the field.
Conclusion
This study shows the potential of using machine learning to accelerate the development of drug delivery technologies, specifically long-acting injectable (LAI) drugs. ML models were used to predict the release of drugs from advanced systems, and the insights gained from the models were used to guide the design of new formulations.
The study found that tree-based models such as Light GBM were more accurate for predicting drug release in this dataset. Although the dataset was small, it is expected that as ML becomes more widely used in drug formulation, larger datasets will become available, leading to the increased utility of neural networks.
Implementing tree-based models in the development of LAI can help reduce time and cost. The study also demonstrated a data-driven approach for designing a promising LAI for the drug olaparib, but it needs further optimization to be efficacious in vivo. Altogether, this research highlights a promising application of ML in drug formulation development and serves as a proof of concept that can aid in the development of more advanced and accurate ML approaches for designing new drug formulations.
Article Source: Reference Paper | Reference Article
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.