Researchers from Scripps Research and Japan have discovered how a protein’s “poisoned” form can set off a chain of events promoting specific cancers’ growth. The research findings were published in Nature Communications. A potential drug that could return the protein to its original state was developed as a result of this study. The drug significantly slowed or stopped tumor growth when it was tested on mice with colon cancer.
Chemically “poisoned” proteins
Proteins that have undergone changes as a result of contact with specific toxins or chemicals are referred to as chemically “poisoned” proteins. These changes impact how cells behave due to the modifications in the structure and function of the protein.
The molecular mechanism behind cancer formation
There is a connection between the environment, genes, and cancer formation. Understanding and targeting this connection could be a valuable approach to combating cancer.
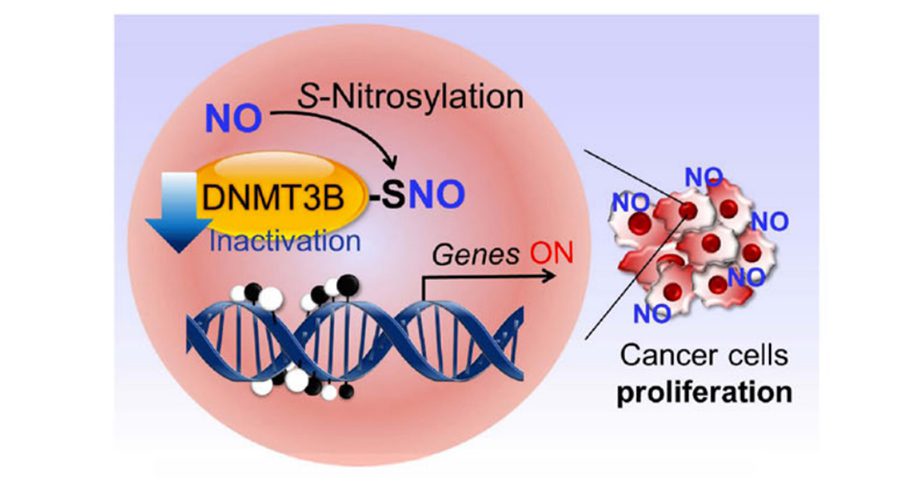
Earlier research by the Scripps Research Group revealed a mechanism known as protein S-nitrosylation. This procedure takes place when a molecule associated with nitric oxide binds to sulfur atoms in proteins, changing the way in which they normally function. The body produces Nitric oxide on its own when there is inflammation. However, it can also be produced in case of exposure to cigarette smoke, smog, and the nitrates and nitrites present in processed meats may also lead to its accumulation. S-nitrosylation has been linked to Alzheimer’s, Parkinson’s, Lewy body dementia, ALS, and some forms of autism.
Genes are activated and deactivated by DNA methyltransferase (DNMT), a key enzyme in epigenomic regulation. These proteins have the ability to chemically tag a segment of DNA with a methyl group, preventing the activation of nearby genes. The loss of these methyl “silencers” in some cancer types results in an irregular activation of genes linked to tumor development and dissemination.
Blocking methylation can result in genes activating at the wrong times, which is a major factor in the development of some cancers. The main reason behind this phenomenon remains a mystery.
The researchers showed that DNA methyltransferase 3B (DNMT3B) loses its capacity to add methyl groups to DNA when it is S-nitrosylated. This reaction can be brought about by high nitric oxide (NO) levels, which make some cancer-causing genes active. The findings suggest that DNMT3B can change into a cancer-promoting form in response to processed meats, air pollution, cigarette smoke, and inflammation, all of which are linked to some types of cancer. It appears to be a dangerous DNMT3B variant.
The team showed they could change the expression levels of 173 different human genes by “poisoning” DNMT3B. One of these genes, Ccnd2, was previously connected to human colon and gastric cancer development.
The collaborating scientists at the Japan group developed a drug that could prevent the S-nitrosylation of DNMT3B without affecting its normal operation or the S-nitrosylation of other proteins. As a result, even in the presence of high NO concentrations, DNMT3B would not change into a “poisoned” state.
In laboratory experiments, the research team found that the drug DBIC could prevent the development of isolated precancerous colon cells into full-blown colon cancer. Furthermore, DBIC effectively prevented tumor development in mice predisposed to colon cancer, even in the presence of elevated NO levels brought on by inflammation.
According to the researchers, the S-nitrosylation of DNMT3B may be related to several cancers, including breast and brain cancer. The complete list of genes impacted by S-nitrosylated DNMT3B will be investigated further.
Conclusion
The current study demonstrates that SNO-DNMT3B inhibits its enzyme activity in cell-based assays, but S-nitrosylation of DNMT1 has no impact on the enzyme’s activity in either in vivo or cell-based models of colon cancer. Additionally, the research offers a special DNMT3B regulator that can specifically reduce SNO modification without affecting the enzyme’s functionality. This chemical regulator, known as DBIC, has therapeutic potential because it significantly reduces tumorigenesis and cell proliferation in vivo. Previous studies have highlighted the importance of NO and epigenetic control in tumorigenesis, but their relationship has been hazy. The research work establishes a mechanistic connection between these occurrences and provides insightful therapeutic information.
Article Source: Reference Paper | Reference Article
Learn More:
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.