In a major scientific breakthrough, researchers at the University of Chicago have succeeded in creating a comprehensive model of the nuclear pore complex with the HIV-1 virus capsid. This sheds new light on the complex processes viruses like HIV use to enter host cell nuclei and replicate.
HIV-1 transports genetic material into the nucleus through nuclear import and viral capsid uncoating, two essential stages in the life cycle. The process is facilitated by the nuclear pore complex (NPC), where complete capsid transit is frequently accommodated in the central channel. The specific mechanisms behind this process are still unknown. This study published in PNAS looks at the molecular mechanisms that control capsid translocation via the central channel by interacting with the HIV-1 capsid and the NPC. The transfer of the capsid to the central channel from the cytoplasmic side of a human NPC is the main subject of the investigation. The compatibility of capsid form and channel dimension, along with the correct orientation of the capsid, drive the translocation dynamics. Additionally, the study finds that docked intact capsids exhibit associated striated patterns of lattice disorder, which are probably connected to intrinsic capsid elasticity. According to the study, the capsid’s inherent “elasticity” may aid in stress adaptation and structural integrity during translocation.
Nuclear pore complexes, which operate as a gate for the movement of cargo between the cytoplasm and nucleus, control nuclear entry in intact HIV-1 capsids. In order to facilitate capsid passage, the central channel dynamically expands, showcasing its adaptable nature for big loads. Lattice disorder with striated patterns is produced by stress brought on by channel confinement and uncondensed genetic material, signifying its “elasticity.” This study shows that modifying the lattice elasticity of capsids can be a useful approach in the creation of antiviral medications since it can hinder viral infection and inhibit nuclear import.
The viral genome is transported into the nucleus as part of a replication cycle that HIV-1 uses to propagate infection. Enclosing and shielding genomic RNA and essential replication-related enzymes is the capsid, which is made up of 1,000–1,500 copies of capsid proteins. Typically measuring 120 nm in length and 60 nm in breadth, the capsid cone is a hexameric lattice consisting of 12 pentamers. Capsid nuclear import is facilitated by the nuclear protein complex, which is encased in the nuclear envelope. Early structural studies found that the diameter of the NPC core channel was approximately 40 nm. Uncoating happens before integration is close to the genomic integration site, reverse transcription takes place within the intact capsid, and intact capsids are carried through the nuclear pore, according to recent findings. Techniques like electron microscopy and cryo-ET validate this observation. The percentage of capsids that can translocate into and via the nuclear pore, as well as the characteristics of the capsid lattice during passage into the NPC central channel, are unknown. Ultrapotent inhibitor medications may also target the capsid’s nuclear import stage, allowing the capsid to rupture prematurely before nuclear entry, preventing the release of genetic material, and decreasing infectivity.
Understanding Nuclear Pore Complex (NPC)
One of the largest protein assemblies in the cell is the NPC, which has a molecular weight of about 120 MDa and can contain up to 1,000 proteins. A thorough understanding of hitherto undesignated complicated parts is now possible because of several recent studies that have established the structure of the human NPC in unprecedented depth. The NPC’s constituent proteins are referred to as nucleoporins (NUPs). In an eightfold rotational symmetry, NUPs assemble to create hetero-oligomeric complexes that form three stacked concentric rings, each with eight spokes. The Y-complex, when oligomerized head-to-tail, forms two concentric and slightly displaced eight-membered rings. This is the building block of the outer cytoplasmic ring (CR) and nuclear ring (NR).
In the same way, an inner ring (IR) complex’s single spoke is made up of several copies of NUPs. Significantly, the IR complex’s linker NUPs provide flexibility in the spokes’ relative arrangement by connecting neighboring spokes. The nucleocytoplasmic cargo exchange is dependent on FG-NUPs (phenylalanine-glycine nucleoporins) that are intrinsically disordered and attached to the NPC scaffold. It is known that several FG-NUPs at the CR, IR, and NR interact with the HIV-1 capsid.
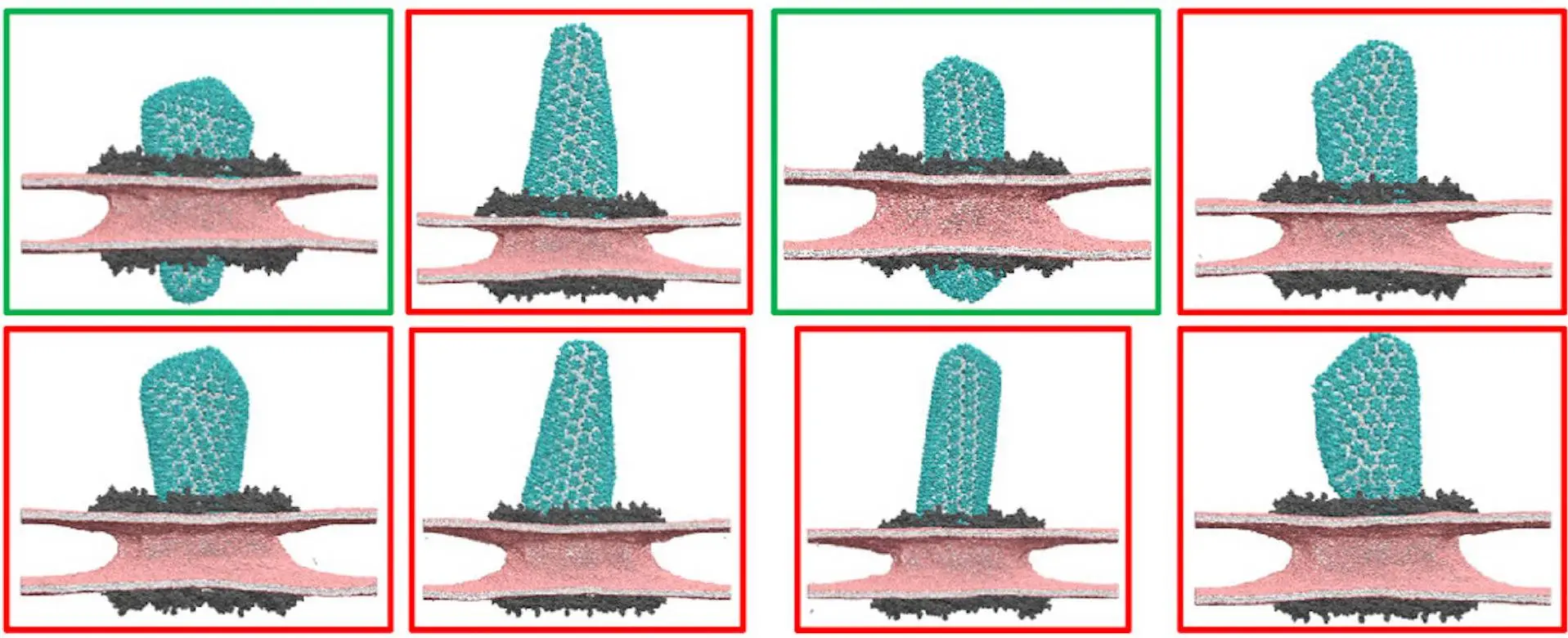
Orientation Dynamics
The spatial and temporal size of the multistep translocation process makes simulations of membrane-embedded NPC with capsid atomistic detail impractical today and for the foreseeable future. Over a billion atoms would be needed. Alternatively, physically sound molecular dynamics (MD) simulations of large protein complexes at relevant scales for typical biological activities can be achieved by the use of methodically constructed “bottom-up” coarse-grained (CG) models. In this case, “bottom-up” means that underlying atomistic interactions are methodically used to generate the CG molecular model and interactions. Put differently, the CG model and interactions that are created are intended to accurately mimic the molecular behavior that is observed in atomistic simulations when those simulations are precisely projected onto a coarser representation.
In order to comprehend large-scale viral processes, such as capsid restriction, immature Gag assembly, and SARS-CoV-2 entrance, this work makes use of CG MD simulations. From the high-resolution structure of human NPC, the researchers develop a bottom-up CG molecular model and interactions of NUP monomers and subcomplexes. The researchers also developed a CG molecular model of the HIV-1 CA monomer and the CG associative connections between CA-CA and CA-FG. The human NPC composite model, which is composed of NR, IR, NUPs with capsid-binding FG-repeats at the IR, and outer CR and NR are embedded in a dilated and constricted membrane. The CG models incorporate the solvent’s effects into the interactions, making them “solvent-free” models.
Linking Simulation to Real-world Observations
This study investigates the translocation dynamics of HIV-1 capsid into the NPC central channel using CG MD simulations. The investigation centers on the interactions between three distinct capsid morphologies—cone-shaped, pill-shaped, and square—and the central channel. Constricted NPC prevents all capsid morphologies from moving, but dilated NPC permits the transfer of cone- and pill-shaped capsids. The incompatibility of channel size and capsid architecture is the main obstacle to nuclear entrance. Precise striated patterns of lattice disorder are revealed by analyzing viral capsid structures at the NPC central channel; these patterns symbolize both capsid flexibility and fragility. The viral genomic complex inside capsids docked at the NPC is modeled to resemble reverse transcription initiation. The structural fragility of the pill-shaped capsid is greatly enhanced by the genetic complex as compared to the more commonly occurring cone-shaped capsid in its uncondensed state.
Conclusion
The study looks into the nuclear import of HIV-1 capsids into the NPC central channel, which is an essential part of big cargo transport that works well. Computer simulations are used in the study to comprehend the mechanisms controlling capsid docking and translocation into the central channel. Effective translocation is contingent upon the capsid approach’s orientation, as energetic contributions cannot break through the first steric barrier due to its high level. When moving towards the center channel, the capsid lattice is disturbed, resulting in disordered domains at the lattice. The study also looks at how the antiviral small molecules PF74 and Lenacapavir affect the intrinsic elasticity of the capsid, stiffening the lattice and causing it to break prematurely during nuclear import. The intricate human NPC has eight capsid-binding FG-NUPs and eight cytoplasmic filaments that make up the complex human NPC. Changing the flexibility of the capsid may be a useful antiviral strategy since it lessens the chance that complete capsids will translocate inside the NPC and decrease infectivity. Future investigations may explore how host factors regulate the dynamics of capsid passage from the NPC central channel to the interior of the nucleus and modulate the capsid structural integrity.
Article source: Reference Paper | Reference Article
Follow Us!
Learn More:
Deotima is a consulting scientific content writing intern at CBIRT. Currently she's pursuing Master's in Bioinformatics at Maulana Abul Kalam Azad University of Technology. As an emerging scientific writer, she is eager to apply her expertise in making intricate scientific concepts comprehensible to individuals from diverse backgrounds. Deotima harbors a particular passion for Structural Bioinformatics and Molecular Dynamics.