Scientists from the University of Helsinki, Broad Institute, and Harvard Medical School have revealed the human genetic epidemiology of the COVID-19 disease caused by SARS-CoV-2. The study indicates that the genetic association studies have been particularly fast in conveying new genetic signals underlying COVID-19 severity and disease susceptibility and can advance the treatment significantly.
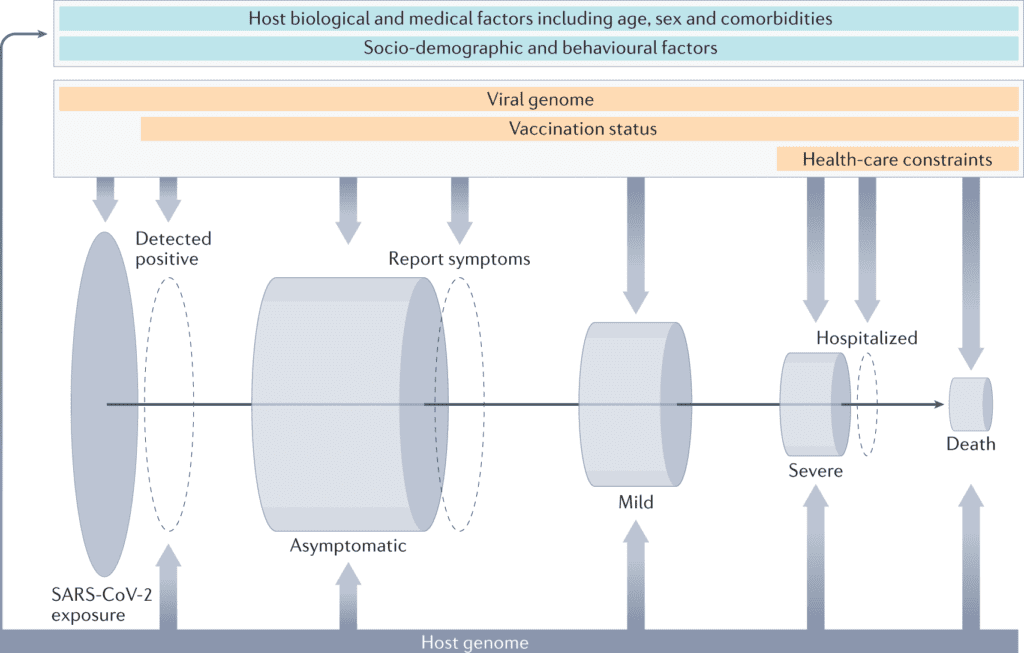
Image Source: The human genetic epidemiology of COVID-19
Human genetics can inform the biology and the epidemiology regarding the coronavirus disease that emerged in 2019 (COVID-19) by pointing at causal mechanisms that explain why a few individuals get severely impacted by the disease upon infection by the SARS-CoV-2 virus.
Large-scale genetic association studies, including both rare and common genetic variants, have utilized different study designs and multiple disease phenotype definitions to recognize several genomic regions related to COVID-19.
Alongside a huge number of follow-up studies, these discoveries have expanded how scientists might interpret disease etiology and gave routes for the management of COVID-19.
Significant emergent opportunities include the clinical translatability of genetic risk prediction, the repurposing of existing drugs, the investigation of variable host impacts of different viral strains, the study of inter-individual variability in vaccination response, and the understanding of long-term results of SARS-CoV-2 infection.
Apart from the current pandemic, these transferrable opportunities would probably influence the study of numerous infectious diseases.
The SARS-CoV-2 Virus and the Outcome of the Sustained Hyperinflammation
The SARS-CoV-2 virus arose towards the end of 2019 and spread quickly across the world, with the WHO announcing a worldwide pandemic on 11 March 2020.
This new betacoronavirus had not been seen previously, however it is connected with the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) coronaviruses.
Scientists currently know that SARS-CoV-2 utilizes the human ACE2 receptor for viral entry, first infecting and replicating in epithelial cells in the nasopharynx and subsequently accessing the distal alveolar space.
The virus is recognized by the immune cells through pattern recognition receptors, noticeably by members from the Toll-like receptor group, for example, TLR3 and TLR7, which promote the synthesis of type I interferons, and by cytoplasmic RNA sensors retinoic acid-inducible gene I (RIGI; otherwise called DDX58) and interferon-induced helicase C domain-containing protein 1 (IFIH1; otherwise called MDA5) inducing type I/III interferon responses.
Secreted type I interferons signal using interferon receptors (IFNARs) to switch on Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2) and, thus, promote the expression of interferon‐stimulated genes, for example, oligoadenylate synthetase 1 (OAS1), OAS2 and OAS3.
Severe ailments of COVID-19 infection include dysregulation of the immune response, resulting in insufficient or delayed type I interferon response.
At last, sustained hyper inflammation brings about increased immune infiltration in the lungs, a decrease in the alveolar lacunar space, cell death by apoptosis, and lung fibrosis.
Role of Human Genetics as a Risk Factor
COVID-19 manifests with a wide scope of symptoms and degrees of severity. Although most cases are currently known to be asymptomatic or mild, a few patients develop a severe type of disease that results in intense respiratory distress syndrome and consequent multi-organ complications.
Disease severity is correlated with numerous risk characteristics, including older age, male sex and smoking, different clinical comorbidities, such as being obese or immunocompromised and clinical biomarkers, autoantibodies to type I interferons cytokines, and inflammation markers.
At the beginning of the pandemic, it was at that point noticed that these clinical factors didn’t completely explain the variability in COVID-19 disease severity among individuals, and extreme cases were observed among younger individuals without apparent past pre-conditions, sometimes clustering in families, suggesting a role for human genetics as a risk factor.
Exploring the Infection Susceptibility and Disease Severity of COVID-19
Finding host genetic factors for infection susceptibility and disease severity is significant because it prompts a better understanding of the viral infection, the pathophysiological changes that occur owing to the disease, and the discovery of potential drug targets.
Likewise, it can reveal insight into the causal relationships between risk factors, biomarkers, and disease outcomes and illuminate prevention strategies.
Notable instances of effective human genetic studies of infectious diseases include the identification of the CCR5Δ32 mutation for protection against HIV infection and the protection against Plasmodium falciparum infection (malaria disease) in people who are heterozygous carriers of a sickle cell allele of the hemoglobin-β (HBB) gene.
Contrasted with other common complex diseases, studying the human genetics of infectious disease represents extra difficulties, including uneven exposure to the virus inside a populace, the differential treatment of patients with the severe disease under a pandemic crisis, and the execution and uptake of vaccination programs.
In any case, the current worldwide expertise in the generation and analysis of human genetic data has considered rapid large-scale studies in host genetics of COVID-19.
In this study, the scientists give an outline of current study designs enabling the discovery of human genetic variation related to COVID-19, with a focus on large scale populace based association studies, the genetic findings made up to this point, and what the individuals have realized as far as biology and general health are concerned.
At last, the scientists give a portion of the vital challenges ahead for the field in this moving pandemic and beyond.
Increasing Phenotypes of COVID-19 and Post COVID-19 Study
Most genetic studies of COVID-19 have focused on pinpointing factors that make a few people more susceptible to the SARS-CoV-2 infection and explaining why others develop severe symptoms.
Nonetheless, with steadily growing comprehension of the disease and the information gathered, future genetic studies might extend to researching, at scale, specific symptoms related to the infection or severe comorbid conditions, for example, multisystem inflammatory syndromes.
Besides, a few people who have contracted COVID-19 experience long-term symptoms that might bring about a significant health burden in the years to come.
There is enormous variability in the symptoms experienced by those impacted by post (long) COVID-19. Human genetics can be helpful in this setting since some of the post-COVID-19 side effects have been directly or indirectly studied by Genome-wide association studies (GWAS). For instance, one could test the hypothesis that COVID-19 accelerates existing genetic predispositions to a portion of the symptoms.
Along with the observational epidemiological analysis, Mendelian randomization (MR) can be utilized as an additional pillar of support to triangulate evidence of a causal relationship between COVID-19 and downstream consequences.
Worldwide networks, for example, the COVID-19 Host Genetics Initiative (HGI), can have a vital influence in such endeavors since they unite studies with different designs, incorporating biobank studies with longitudinal medical data pre-and post-infection and direct-to-consumer studies that can capture self-reported symptoms on an enormous number of individuals.
COVID-19 Host Genetics Outlook
Continued investigations concerning host genetic factors that add to severe COVID-19 and susceptibility to SARS-CoV-2 viral infection will be fundamental to expand the possibilities of tracking down new therapeutic avenues to treating the infection, whether it be through drug repurposing or the longer-term endeavor of new drug development.
These discoveries should be integrated with multi-omics results to give more clear biological insights. Concerning some other complex diseases, genetic risk prediction is probably going to add value to clinical risk prediction in a hospital setting for the identification of patients who are bound to develop severe symptoms, and along these lines, continued efforts in the identification of risk factors and the development of predictive biomarkers are warranted.
The Endpoint
Host genetics isn’t the sole key to figuring out the code to fruitful and effective treatment of COVID-19, yet with the continuation of open science and partnerships between academics, industry, healthcare providers, and policymakers, we will hopefully get to see huge leaps toward that aim soon.
Genetic association studies have been particularly fast in conveying new genetic signals underlying COVID-19 severity and disease susceptibility. On a sobering note, these discoveries limitedly affect the management of the COVID-19 pandemic thus far, and hopefully, the next phase of the pandemic will see more utilization of human genetics results and better functional insights.
Article Source: Niemi, M.E.K., Daly, M.J. & Ganna, A. The human genetic epidemiology of COVID-19. Nat Rev Genet (2022). https://doi.org/10.1038/s41576-022-00478-5 DOI: https://doi.org/10.1038/s41576-022-00478-5
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Tanveen Kaur is a consulting intern at CBIRT, currently, she's pursuing post-graduation in Biotechnology from Shoolini University, Himachal Pradesh. Her interests primarily lay in researching the new advancements in the world of biotechnology and bioinformatics, having a dream of being one of the best researchers.