New research from Vanderbilt University suggests that a protein involved in the production of high-density lipoprotein (HDL) works differently than previously believed.
High-density lipoproteins (HDL) are a type of protein that helps transport cholesterol throughout the body. HDL particles have a higher concentration of cholesterol than low-density lipoproteins. The HDL cholesterol or “good cholesterol” carries fat and cholesterol away from artery walls and may reduce or prevent atherosclerosis and coronary heart disease.
The researchers investigated the role of ABCA1 in transporting fatty molecules between the cell’s plasma membrane and HDL using computer simulations and cell culture studies. Researchers published the findings in the journal Nature Communications, which suggests that, as opposed to previous assumptions, ABCA1 extracts phospholipids from the outer surface of the plasma membrane, not the inner surface.
The model proposed in the study suggests that ABCA1 transports extracellular lipids using a unique mechanism, which differs substantially from mechanisms related to other members of this transporter family. It is surprising to discover that the ABCA transporter superfamily exhibits remarkably diverse substrate transport characteristics.
The ABCA1 transport mechanisms are important because they provide insights into possible pathways for promoting ABCA1-dependent phospholipid and cholesterol efflux from macrophages that contain cholesteryl ester, which is integral to the development of atherosclerotic lesions.
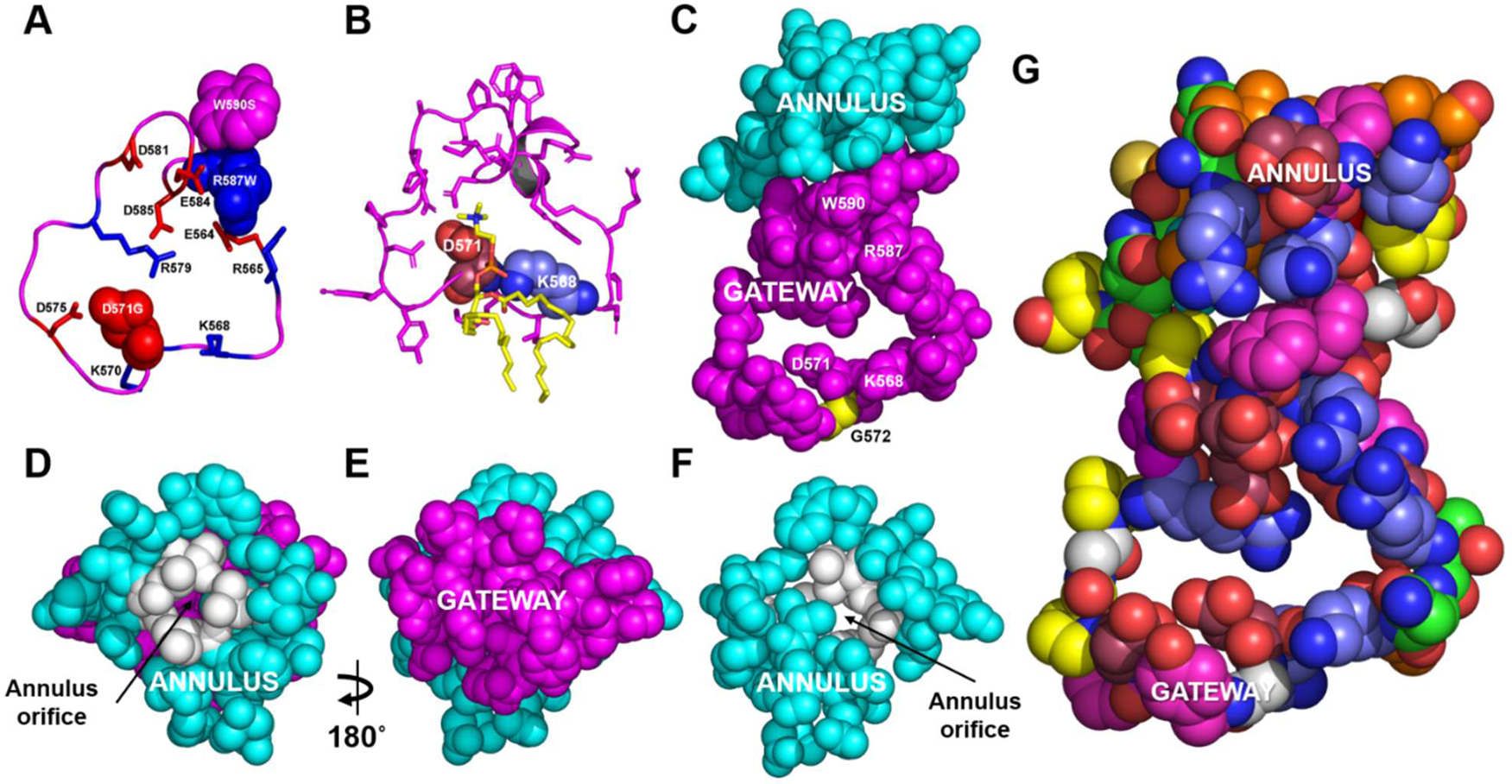
Researchers used coarse-grained molecular dynamics simulations to demonstrate that ABCA1 domains in the plasma membrane remove PL from its outer leaflet. Following diffusion into ABCA1’s outward-open cavity, PL extracted by the gateway passes through an annulus orifice in the transporter’s extracellular domain, forming an elongated hydrophobic tunnel.
It was found that engineered mutations in the gateway and annulus strongly inhibit ABCA1 from exporting lipid without affecting its expression on the cell surface.
Final thoughts
In the current study, it has been found that ABCA1 extracts lipid from the outer surface of the plasma membrane and forces it into a long hydrophobic tunnel through its gateway and annulus. This differs from the alternating access model, which proposes that ABCA1 flips PL substrates from the inner leaflets to the outer leaflets of the membrane. In accordance with the model presented in the study, ABCA1 lacks the charged amino acid residues in the transmembrane domain found in the floppase members of ABC transporters.
Learn More:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar
- Dr. Tamanna Anwar