There’s still a lot to discover about how the HIV-1 virus affects human cells. Scientists believe it gets past our immune system’s defenses and into white blood cells, where it delivers its genetic payload and hijacks the cell’s transcription machinery, which produces copies of viral RNA and new HIV-1 viruses. Though, many details remain unclear.
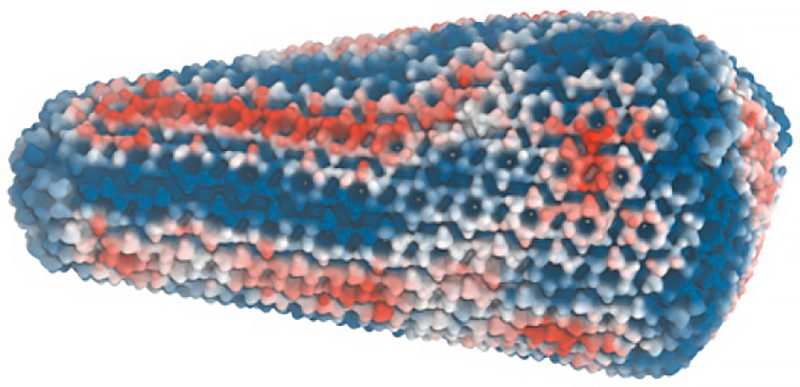
Image Source: https://www.tacc.utexas.edu/-/supercomputing-helps-reveal-weaknesses-in-hiv-1-armor-
A fullerene-shaped capsid transports genetic material deep into the nucleus of an infected cell, allowing HIV-1 to replicate. The HIV-1 mature capsids are transiently steady complexes that self-assemble along the viral genome during maturation, and uncoat to release preintegration complexes that archive a double-stranded DNA copy of the virus in the host cell genome.
In 2021, a key scientific finding shed some light on the riddle, revealing that the viral capsid, a protein envelope that protects the virus’s RNA genome, survives intact all the way inside the target cell’s nucleus. Finally, the capsid must remain stable long enough to deliver its lethal genetic cargo to the cell’s nucleus. However, it must eventually disintegrate in order to release its genetic material. Scientists are still puzzled as to how and why the HIV-1 viral capsid might become unstable.
The Frontera supercomputer at The University of Texas at Austin has helped scientists better understand how the HIV-1 virus infects by creating the first realistic simulations of its capsid, complete with proteins, water, genetic material, and a key co-factor called IP6 that was recently discovered to help stabilize and form the capsid.
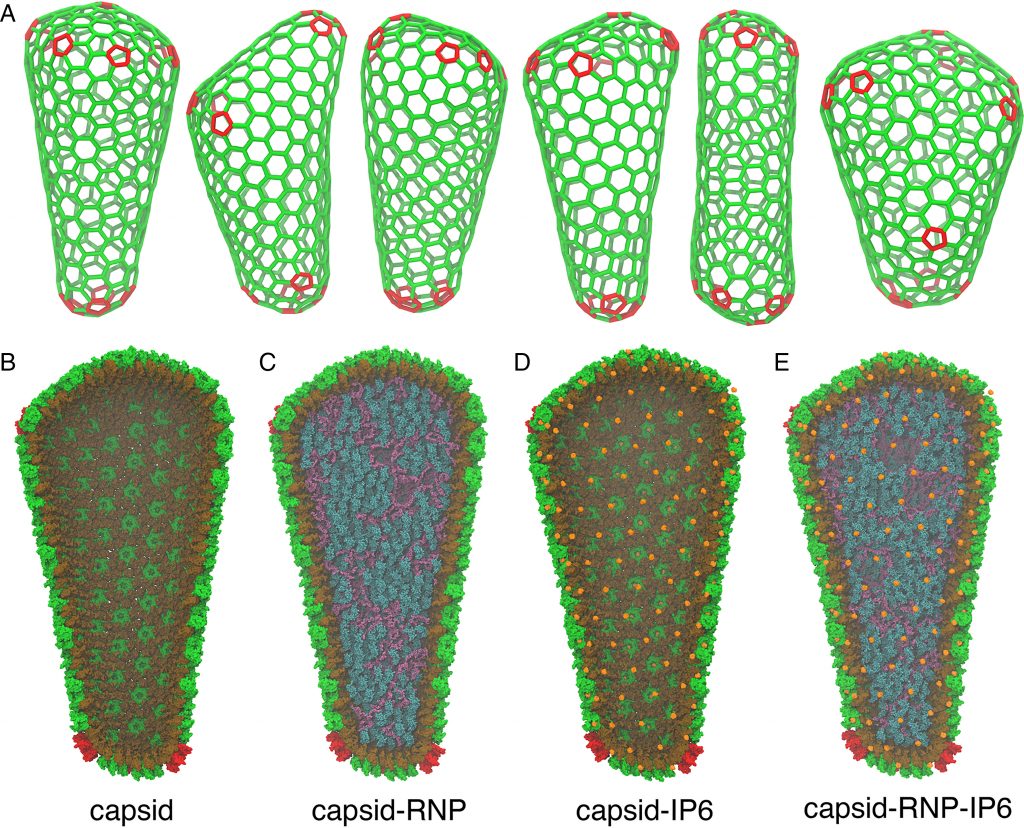
Image Source: Strain and rupture of HIV-1 capsids during uncoating
According to Gregory Voth, the Haig P. Papazian Distinguished Service Professor at the University of Chicago, these massive simulations and the analysis the researchers conducted revealed the flaws in the HIV-1 viral capsid’s armor. Voth is the lead author of a study on HIV-1 capsids that was published in the Proceedings of the National Academy of Sciences in March 2022.
Voth and his group began with data from genuine viruses gathered by co-author John Briggs’ group at the Max Planck Institute of Biochemistry’s Department of Cell and Virus Structure. They built an all-atom molecular dynamics simulation of the HIV-1 capsid based on the experimental data that surpassed 100 million atoms.
Striations on the capsid are visible in the study’s photos, indicating stress strain. They can pinpoint where the protein lattice is compressed or extended and enduring excruciating strain, indicating that stress is not evenly spread.
That’s important because the researchers were able to link the strain patterns in the lattice to how the capsids really break apart, stated Voth. The pressure exerted within the HIV-1 viral capsid as it undergoes reverse transcription and begins generating DNA should make the stress-strain striations susceptible.
Additional cryo-electron tomography investigations by one of the study colleagues, Owen Pornillos, Department of Molecular Physiology and Biological Physics, University of Virginia, showed that the stress-strain patterns fit well with how the capsid breaks apart, according to the scientists.
According to Voth, this is the most accurate HIV capsid simulation study yet. The researchers also detected that the proteins that pack into this virus capsid had somewhat different conformations than in simpler crystal structures or in vitro reconstitutions.
Previous work by Juan R. Perilla of the University of Delaware and the late Klaus Schulten of the University of Illinois at Urbana-Champaign, as well as coworkers, was published in Nature in 2017. On the Blue Waters supercomputer, those researchers created the first model of the HIV-1 capsid. It lacked the inner genetic material, the IP6 co-factor, and wasn’t created from cryo-electron tomography data of genuine viral capsids, as groundbreaking as it was at the time.
The vulnerabilities in the armor of the HIV-1 virus capsid were revealed by these very big simulations and the analysis we did.
Gregory Voth, University of Chicago
All of these missing elements were incorporated into the new model. This work is a huge step forward in realistic modeling, and the researchers hope it will help to better comprehend this capsid, according to Voth.
Gilead Pharmaceuticals is developing the HIV-1 drug lenacapavir by making the viral capsid more brittle, which interferes with the viral capsid’s essential break-up stage before releasing its genetic material.
Voth explained that what their team is doing now is to see whether they can understand how drugs interact with this virus capsid and if they can develop novel drugs, given the diverse character of the virus capsid.
Furthermore, the idea of a one-two punch pharmaceutical, in which one drug molecule binds to the capsid protein lattice, allowing another drug molecule to bind, has grabbed the attention of drug designers.
Supercomputers combined with the methods we developed have helped reveal essential elements of the HIV-1 virus that are experimentally extremely difficult to probe at present. I don’t think we could have easily done those simulations anywhere else but on Frontera. It’s a very valuable resource for us.
Voth
Story Source: Alvin, Y., Y., L. E. M., G., B. J. A., K., G.-P. B., Owen, P., & A., V. G. (2022). Strain and rupture of HIV-1 capsids during uncoating. Proceedings of the National Academy of Sciences, 119(10), e2117781119. DOI: https://doi.org/10.1073/pnas.2117781119 https://www.tacc.utexas.edu/-/supercomputing-helps-reveal-weaknesses-in-hiv-1-armor-
Learn More About Bioinformatics:
Top Bioinformatics Books ↗
Learn more to get deeper insights into the field of bioinformatics.
Top Free Online Bioinformatics Courses ↗
Freely available courses to learn each and every aspect of bioinformatics.
Latest Bioinformatics Breakthroughs ↗
Stay updated with the latest discoveries in the field of bioinformatics.
Dr. Tamanna Anwar is a Scientist and Co-founder of the Centre of Bioinformatics Research and Technology (CBIRT). She is a passionate bioinformatics scientist and a visionary entrepreneur. Dr. Tamanna has worked as a Young Scientist at Jawaharlal Nehru University, New Delhi. She has also worked as a Postdoctoral Fellow at the University of Saskatchewan, Canada. She has several scientific research publications in high-impact research journals. Her latest endeavor is the development of a platform that acts as a one-stop solution for all bioinformatics related information as well as developing a bioinformatics news portal to report cutting-edge bioinformatics breakthroughs.





