Despite the challenge of scaling for large-scale screenings, advances in computational structural biology, like AlphaFold-Multimer, have greatly improved the understanding of protein-protein interactions across entire proteomes, enabling large-scale screenings and elucidating system properties within or between organisms. Here, researchers provide AlphaFastPPi, a pipeline based on AlphaPulldown and AlphaFold-Multimer. AlphaFastPPi is a simplified pipeline that uses fewer resources than previous approaches, increasing efficiency. This is accomplished by reducing the necessary minimum number of models from five to one while keeping a high level of accuracy. This offers a more long-term method for predicting PPIs. Researchers show that this optimization offers a quick, practical, and green approach for PPI predictions without sacrificing the method’s accuracy.
Introduction
Protein-protein interactions (PPIs) are essential for understanding cellular processes such as metabolic pathways, gene regulation, and signal transduction. Additionally, host-pathogen interactions are essential for broader biological mechanisms. Mapping PPIs at the proteome level is leading to significant discoveries in biological systems.
The computer tools AlphaFold-Multimer and AlphaPulldown have transformed the field of protein interaction research. AlphaFold-Multimer models protein polypeptides using GPUs, improving prediction accuracy and speed. Optimized pipelines have been created to speed up the computational workflow. These include quicker multisequence alignment search options and the separation of CPU and GPU for increased efficiency. A Python tool called AlphaPulldown simplifies the process of screening PPIs. However, the high processing time, disc space, and GPU resources needed for large-scale PPI screening continue to be a challenge and may restrict the scalability and accessibility of these tools for large-scale research.
Understanding AlphaFastPPi
A Python tool named AlphaFastPPi simplifies the AlphaFold-Multimer process for PPI screening, making it more accessible to users with little computational power. Cutting down on the number of models produced from five to one preserves computer resources and makes large-scale analysis more viable. AlphaFastPPi is more inclusive and sustainable since it can predict protein interactions on a dataset of intra- and inter-species PPIs. This study shows how AlphaFastPPi may be used to lessen resource waste and environmental effects, making it a more sustainable and inclusive approach.
AlphaFastPPi Pipeline
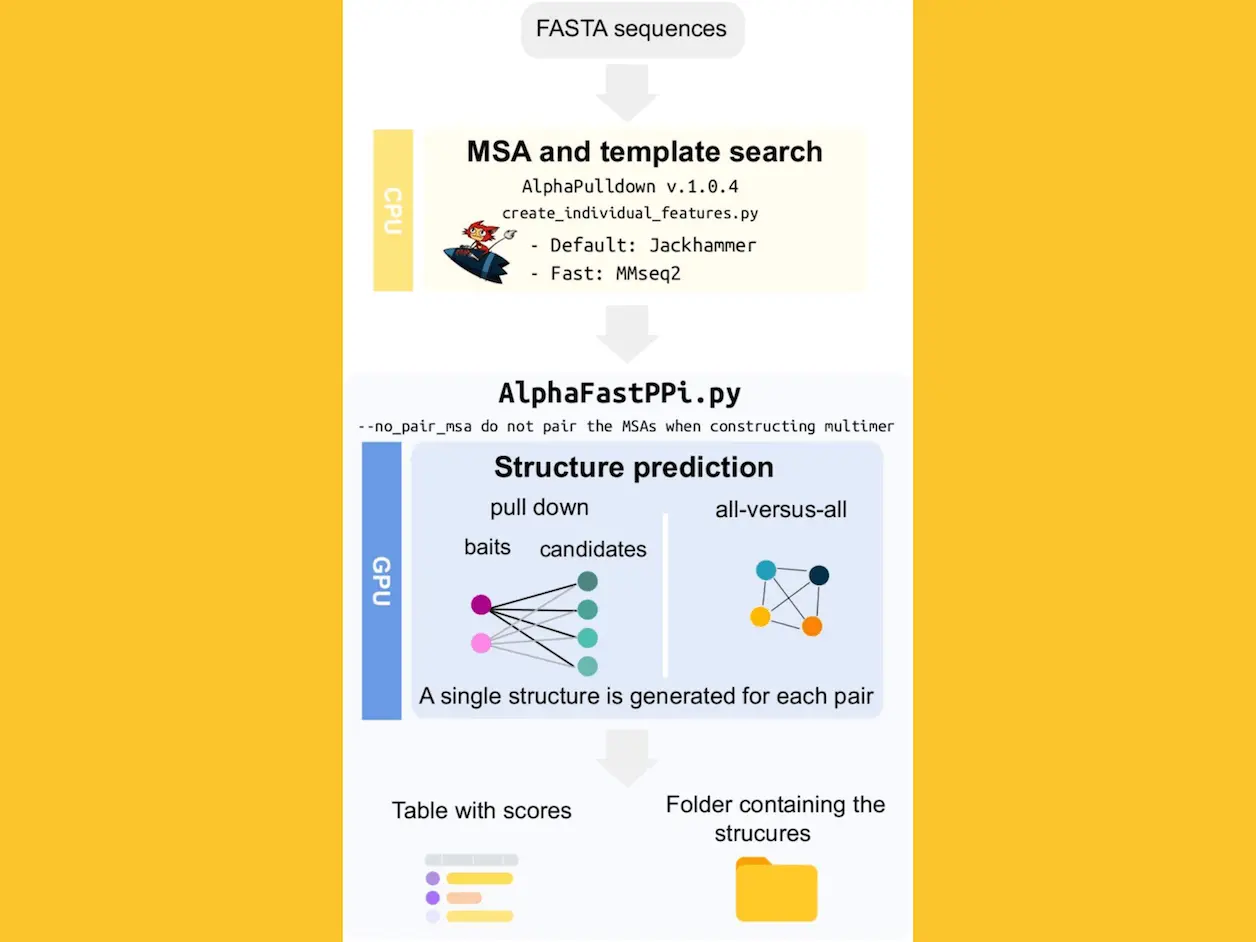
The AlphaPulldown v.1.0.4 script uses CPUs to provide structural template features and Multiple Sequence Alignments (MSA) for protein prediction. When GPUs are available, AlphaFastPPi creates a single model for each PPI; in the absence of GPUs, CPUs are utilized. Unpaired MSAs are employed to improve speed. Two modalities are available with AlphaFastPPi: pull-down and all-versus-all. Users submit a list of proteins as “baits” and another protein as “candidates” when using pull-down mode. AlphaFold-Multimer produces a single model for each pair by comparing each bait protein to every candidate protein. The program generates a single model for each pair in the all-versus-all mode by computing every possible combination of the proteins. For every anticipated PPI, model quality scores are determined and saved for further examination.
One hundred forty-two heterodimeric protein complexes between Arabidopsis thaliana and Serendipita indica, as well as positive PPIs from Mycoplasma pneumoniae, made up the test set that was created using the Negatome database. The positive data set included 286 PPIs and 428 true negatives—selected randomly from non-interacting protein pairs—made up the negative data set.
Methodology of AlphaFastPPi
One model is produced for each protein pair by AlphaFastPPi, whereas the AlphaPulldown modeling stage creates a minimum of five models. Pull-down mode was used to test both approaches on a dataset of protein pairings that had been shown to interact and not interact in experiments. GPU acceleration was not strictly required, but predictions were run on NVIDIA A100 SXM6 64GB GPUs. When screening 856 interactions, AlphaFastPPi saved roughly 4.7 times the GPU hours, or more than 8 days, and 5.0 times the disc space, or more than 2 TB. It was evaluated if the approach could discern between pairs that interacted and those that did not. Though scoring measures such as pDockQ and ipTM have been developed, none of them have been particularly created to answer the question of whether two proteins are interacting or not. When comparing the findings of AlphaPulldown with the highest pDockQ model, no discernible difference was seen between the two approaches. This implies that the discriminatory capacity of differentiating between interacting and non-interacting pairings remains unaffected by reducing the number of predictions from five to one.
On a benchmark dataset, it was discovered that the pDockQ cut-off was superior to ipTM in terms of obtaining specificity and sensitivity in interactions. The results of AlphaFastPPi and AlphaPulldown showed a sensitivity of about 24% and a specificity of over 70%. Specificity increased to over 90% when the pDockQ threshold was raised to 0.5; however, sensitivity decreased to less than 10%. When compared to intra-species interactions, inter-species interactions displayed lower confidence levels. The method’s capacity to differentiate between interacting and non-interacting protein pairs may be improved by massive sampling, but its computational demands make it unsuitable for large-scale studies.
Conclusion
AlphaFastPPi is a simplified and effective method for predicting protein-protein interactions (PPIs) that uses less computer power while still producing predictions of interacting pairs that are competitive. This tool meets the demands of the scientific community in a variety of ways, from those conducting brief preliminary assessments to those engaging in extended studies with limited resources. It provides a quick and efficient way to narrow probable interactions for further investigation. Additionally, it helps to make computational investigations of PPIs more viable.
Article Source: Reference Paper | AphaFastPPi code is freely available on GitHub.
Follow Us!
Learn More:
Deotima is a consulting scientific content writing intern at CBIRT. Currently she's pursuing Master's in Bioinformatics at Maulana Abul Kalam Azad University of Technology. As an emerging scientific writer, she is eager to apply her expertise in making intricate scientific concepts comprehensible to individuals from diverse backgrounds. Deotima harbors a particular passion for Structural Bioinformatics and Molecular Dynamics.