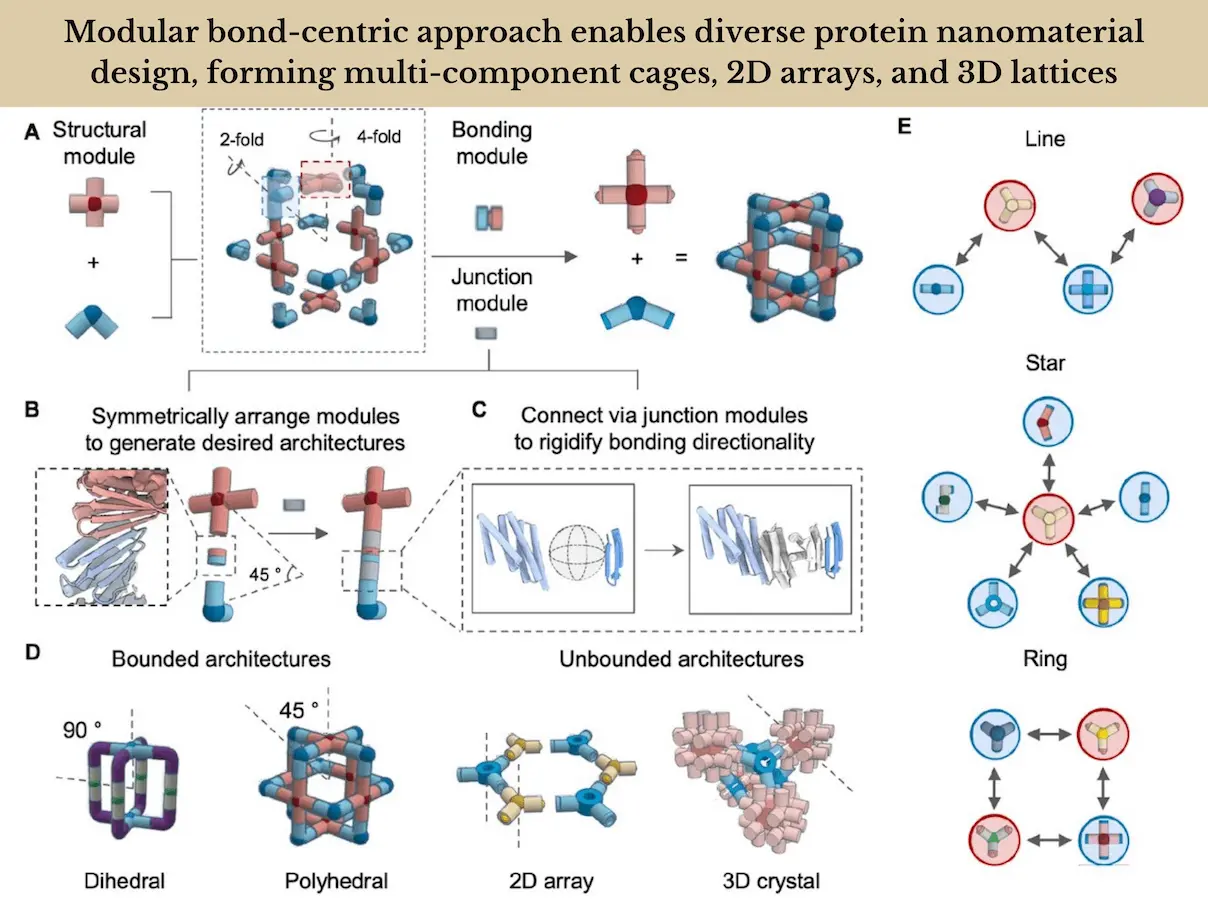
Scientists present a modular bond-centric strategy for designing protein nanomaterials that are motivated by the wide range of chemical configurations that may be produced from a limited number of atomic valencies and bonding interactions. Through the application of basic geometric concepts, researchers from the University of Washington create protein building blocks with regular coordination geometries and bonding interactions that facilitate the assembly of a broad range of closed and opened nanomaterials. Electron microscopy data closely matches the appropriate design models, and experimental characterization verifies the successful production of over twenty multi-component polyhedral protein cages, 2D arrays, and 3D protein lattices, with a high success rate of 10–50%. Due to its modular design, individual building blocks can be assembled with various partners to create unique regular assemblies. This leads to a reduction in the number of parts needed and makes it possible to develop reconfigurable systems.
Introduction
In chemistry, bonding is essential for creating interactions between atoms in both small and large molecules. It is possible to arrange a large number of atoms at precisely defined distances and orientations with predicted interaction strengths using a very small set of atoms and bonding geometries. For stepwise molecular synthesis to work, this modularity is essential. Well-defined nanoscale structures have been produced in supramolecular systems based on comparable bonding ideas. However, because of the intricate sequence-structure links and strong folding cooperativity of proteins, creating protein assemblies through predictable bonding through protein-protein interactions continues to be a substantial difficulty. Robust prediction and design of multi-component architectures beyond cyclic oligomers remain a difficulty, despite advancements in deep learning-based computational approaches. To create internal links in cyclic rings and linear chains, reversible heterodimeric proteins, or LHDs, have been developed. Through large-scale sampling of potential junction geometries produced by fusing helical repeat protein building blocks, the WORMS software facilitates the generation of symmetric assemblies from predetermined cyclic oligomers and bonding modules.
Utilizing LHD Protein Bonds for Assembly
The goal of the research was to create a basic procedure for creating protein architectures using universally compatible building pieces that could be combined to create a vast array of closed and open three-dimensional designs. Researchers reasoned that reversible LHD interfaces might be employed on oligomeric building blocks with suitably matched internal geometry as programmable bonding modules. Aligning building block pairs at particular degrees of intersection between their principal rotational axes could be used to target distinct symmetric designs. Rigid connection adaptors would be needed to guarantee the exact placement and orientation of individual modules because loosely grafted bonding modules can result in poorly defined aggregates or hydrogels.
Design Process: Sampling and Structural Alignment
Researchers investigated the construction of protein assemblies utilizing the established LHD protein bonds in three steps. The building blocks and LHD modules are arranged in space according to the chosen overall architecture, LHD bonding modules, building block cores (typically symmetric homo-oligomers), and degrees of freedom to be sampled in the first step. Gaps are left between the bonding module and building block termini. The next phase involves creating stiff connections between the LHD modules and the building blocks to maintain the necessary relative orientations. Only distinct connections inside an asymmetric unit require explicit generation for symmetric assemblies. Second, in order to stabilize the intended new junction, researchers use backbone sampling to look for backbone arrangements. To do this, researchers either created rigid backbones connecting the bonding and core modules directly using RFdiffusion, a deep generative neural network, or researchers combined pre-designed helical construction blocks using WORMs to generate the necessary geometries. While RFDiffusion is excellent at producing more compact structures that are ideal for developing 2D arrays and 3D lattices, the WORMs technique typically yields extended structures. It is possible to design several partners to co-assemble using a single shared building block through the use of shared bonding modules, resulting in protein-protein interaction networks with various topologies.
Advantages of Bond-centric Strategy
- Protein design has advanced significantly by allowing precise self-assembly of constrained and unbounded nanomaterials. This achievement is credited to the programming of clearly defined directional interactions, which is made possible by fusing generative protein design with reversible heterodimeric interfaces.
- This methodology streamlines the design process by enabling the creation of an extensive array of scalable assemblies from a limited number of reusable building parts. Using customized building blocks and standardized reconfigurable interfaces increases programmability and success rates, which is a benefit over WORMS-based nanomaterial design.
- This method allows for independent control of bonding geometry and interaction strength, extending earlier work to multi-component systems and opening up the possibility of exploring unusual structures and phases with broken symmetry that are difficult to approach using conventional techniques.
- Building block reuse facilitates the quick creation of new architectures from substructures of previously verified assemblies, increasing the success rates of cage designs that make use of these blocks. The C3 component, which can be driven into five different nanocage assemblies depending on the added partner, demonstrates how a single component can form multiple distinct assemblies. This feature not only allows for an economy of code but also opens up opportunities for information storage, as the assemblies populated will depend on the order of addition. The protein assembly networks may also find applications as logic gates that generate different outputs according to other inputs.
Conclusion
The research shows that computational protein design has great promise for creating designer nanomaterials that will soon surpass the capabilities of DNA nanotechnology. Because the designed proteins can be expressed through genetic encoding in a variety of living systems, direct integration of these proteins as structural, signaling, and control units within living cells is a possibility, which might lead to a revolution in cellular computing. Standardized protein subunits should make it easier to create protein assemblies for a variety of uses, much as standardized parts revolutionized industrial manufacturing. These subunits combine according to straightforward guidelines.
Article Source: Reference Paper | The code and notebook used to perform the simulation in this study are available on GitHub.
Important Note: bioRxiv releases preprints that have not yet undergone peer review. As a result, it is important to note that these papers should not be considered conclusive evidence, nor should they be used to direct clinical practice or influence health-related behavior. It is also important to understand that the information presented in these papers is not yet considered established or confirmed.
Follow Us!
Learn More:
Deotima is a consulting scientific content writing intern at CBIRT. Currently she's pursuing Master's in Bioinformatics at Maulana Abul Kalam Azad University of Technology. As an emerging scientific writer, she is eager to apply her expertise in making intricate scientific concepts comprehensible to individuals from diverse backgrounds. Deotima harbors a particular passion for Structural Bioinformatics and Molecular Dynamics.