An endeavor to create new medicinal compounds that can reach great heights, though challenging, remains vital. The advent of computer tools has led to extra creative routes to define and improve molecules within this chemical combinatorial area. Among these achievements is REvoLd, a system developed by researchers from Leipzig University, in collaboration with Vanderbilt University, to address de novo drug design.
So, what exactly is REvoLd, and how does it work?
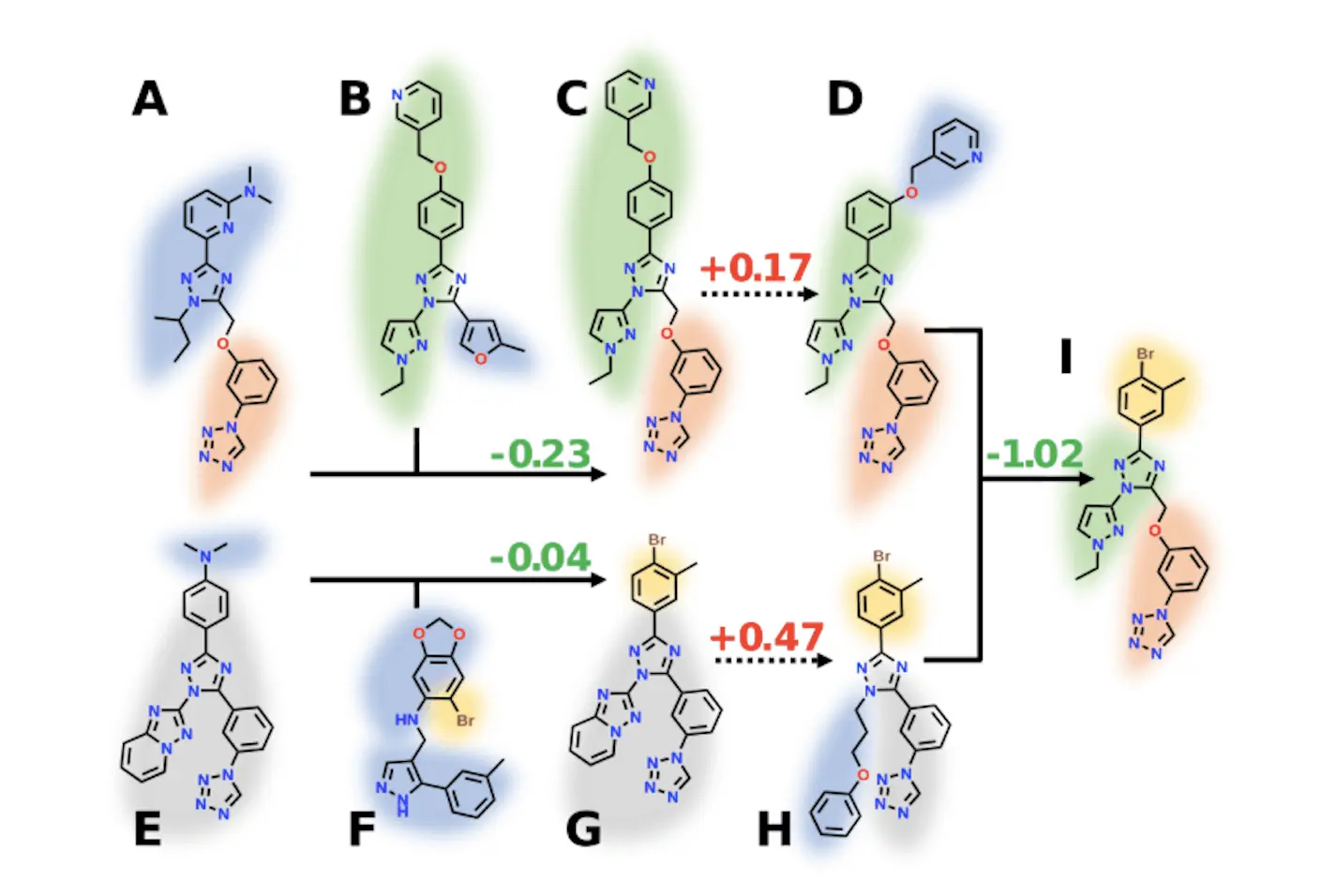
REvoLd is an exhaustive computational method that evaluates the entire chemical compound libraries for this set of drug candidates in addition to the potentially novel drug molecules. The complex drug development environment requires a statistics-based virtual screening method like vHTS due to its vastness of available chemicals. Although computational chemistry constitutes a suitable method of approach to the study of this field, unique roadblocks remain to be identified and resolved for the purpose. Present approaches, Deep Docking, V-SYNTHES, and Targeted Exploration, are attempts, leaving exclusions. Though Galileo’s prospects are promising, they impose a heavy computational effort. As a means, REvoLd is an effective all-around evolutionary algorithm with the unique skills to optimize the candidate compounds from the Enamine REAL database, giving preference to accessible synthesis and demonstrating strong enrichment.
Taking over Darwinian principles
The evolutionary algorithm of REvoLd starts with a random molecule population and applies different selection pressures only when a fitness function is used. This analysis, the most common one that utilizes the hitting-points scoring, comparatively measures the interoperability pegged on a particular target protein and a ligand. A Clone is displayed with a greater fitness grade as a survival of the fittest. The rest, on the other hand, depends on clones’ reproduction, including mutation and crossover.
The program focuses on investigating a narrow space that includes drugs with no potential, such as Enamine REAL space or Otava CHEMriya space. Each thread is profiled by a reaction and a set of fragments; accordingly, the study of billions of possible molecules is enabled.
The strategy starts with the production of the random molecules that are afterward docked to determine their fitness. Selection pressure is also applied among the population, and only the best individuals will be allowed to reproduce to give rise to a new generation. Three selectors are available: ElitistSelector, TournamentSelector, and RouletteSelector would willingly work in consideration with the very degree of determinism in selection.
Reproduction occurs through three child factories: GenesPlay invokes three factories: IdentityFactory, MutatorFactory, and CrossoverFactory. The production lines achieve the alteration of the participants in three ways: through point mutations, response mutations, and crossover events.
The Rosetta docking protocol applies to optimizing the protein-ligand complex by calculating the energy of the generated complex. Finally, it comprises an algorithm including seven alternations that make the history create reproductions in successive chemical searches.
Over and over, by the rule of thumb, this cycle repeats for generations, with the most promising molecules being screened for further investigation. One can adjust the algorithm parameters to the selection type or population size and then tune up the optimization process accordingly.
Finding and analysis
For the research, the authors published REvoLd – a powerful computational method for thoroughly examining big chemical spaces of drug discovery processes. They aimed to overcome bias with bigger ones by using lid_root2 as a reference and fine-tuning ways that helped us to enrich hit rates. REvoLd is the clear winner in all simulations using the actual amount of data, with over 1,600-fold enrichments in some instances compared to random sampling. Answering questions from qualitative research showed that REvoLd used the same mutation and crossover exchange processes that scientists employ to treat diseases. Part of the run-time exploration process was that the scalability increased with the core count, and the larger the chemical spaces needed longer rounds of startup and memory, but it did not affect the algorithm’s capability.
The SUV approach of REvoLd sets itself apart when it comes to docking, among other methods, as its effectiveness in this regard is derived from its ability to combine and aggregate the necessary parameters that reflect attached runs. Alternative operations consisting of Galileo or V-SYNTHES get much lower enrichment in numbers and the same effect as REvoLd with no significant increase in computational burden.
However, Deep Docking and Chemical Space Docking have to account for all molecule information in their approaches, which still demand long docking runs and may become difficult to get along with when the Chemical Space is expanded. Predicted Exploration fails because it assumes that we know the chemistry of binding reactions.
Concluding remarks
REvoLd provides a solution that is quite effective for large libraries that possess several combinations up to billions. While using the prolific evolutionary optimization method, this program finds the best counterparts for this attempt. Results from our benchmark unambiguously disclose that it is superior for sample selection in each of the five tasks. While other methods constantly need protein-ligand re-docking to improve results, the REvoLd strategy is strongly consistent. It produces excellent outcomes after successive runs of dockings, regardless of library size. So far, the comprehensive pipeline can provide target-specific compound libraries utilizing combinatorial chemical libraries, so this prototype removes the need for very extensive and time-consuming docking. In using chemicals from on-request-making services, it is still possible to conduct trials quickly, for example, of drugs recommended by a chemical hit report. So, synthetic accessibility scores are not to be deemed a necessity. A concentrated effort shall be devoted to measuring the docking process in the future. REvoLd’s efficacy is bound to be improved significantly by introducing refinements to docking.
Article Source: Reference Paper | All code used will be available as part of the Rosetta repository on GitHub.
Important Note: arXiv releases preprints that have not yet undergone peer review. As a result, it is important to note that these papers should not be considered conclusive evidence, nor should they be used to direct clinical practice or influence health-related behavior. It is also important to understand that the information presented in these papers is not yet considered established or confirmed.
Follow Us!
Learn More:
Anshika is a consulting scientific writing intern at CBIRT with a strong passion for drug discovery and design. Currently pursuing a BTech in Biotechnology, she endeavors to unite her proficiency in technology with her biological aspirations. Anshika is deeply interested in structural bioinformatics and computational biology. She is committed to simplifying complex scientific concepts, ensuring they are understandable to a wide range of audiences through her writing.
















Github link is broken
Thanks for letting us know! The GitHub link has been updated now. Please try again.