As biologists have noticed, evolution has modified both bacteria and bacteriophages (i.e., viruses of bacteria), making them more complex on a smile’s molecular level. Weissman and his colleagues, Vassalo, Doering, Laub, and Michael (2007), made progress in resolving this molecular ‘cold war’ through this study, which was based on primary research performed at the Massachusetts Institute of Technology. Their work, as described in the publication of Nature, reveals a bacterial protein that changes the structure of mRNA and blocks translation of the viral genome, which enables the termination of virus multiplication. This event has advanced our understanding of mRNA-modifying proteins onboard the ribosomes.
Why Is This Study Important?
It is important to appreciate how bacteria protect themselves from viruses for several reasons. Bacteriophages occur naturally, and they may have therapeutic potential in the field of phage therapy, which is currently under investigation as one of the alternatives in treating antibiotic-resistant complicated infections. This knowledge could help discover new avenues to control or improve bacterial resistance that could be later used for medical purposes. In addition, this study done by MIT’s team helps in providing essential information on an unexplored bacterial mechanism of inhibition of phage reproduction that does not incorporate the phage’s mechanisms.
The Discovery of CmdT: A Novel Defense Mechanism
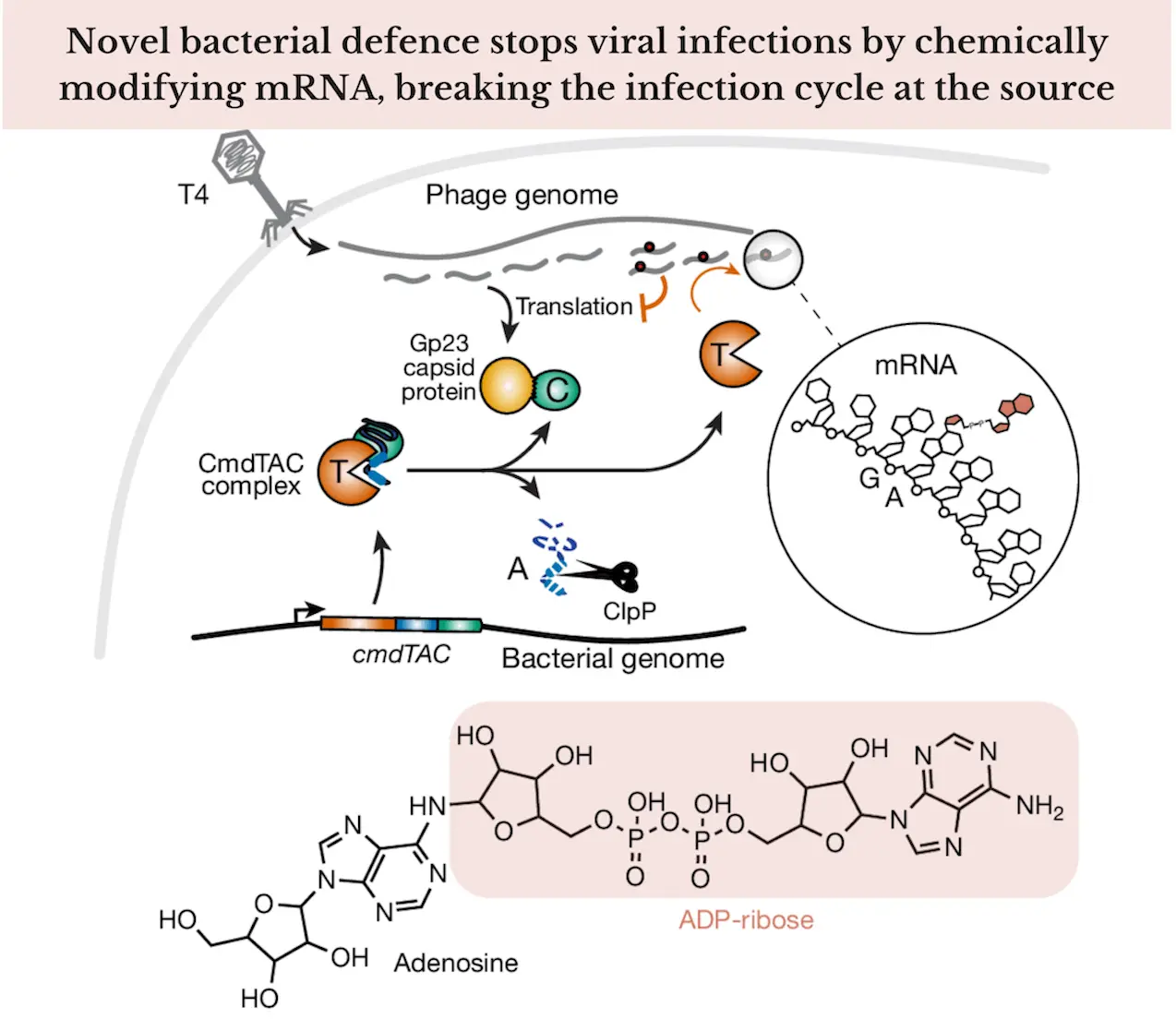
CmdT is one of the focus points of this study, an enzyme that belongs to the group of enzymes called ADP-ribosyltransferase. CmdT is a bacterial-encoded enzyme that is part of a defense system directed against viral mRNA. This type of RNA is known as messenger RNA, which is the key encoding gene, transferring genetic information from the DNA to ribosomes for protein synthesis. When a bacterium is attacked by a phage, the phage utilizes the resources of the bacterial cell, translating its mRNA and forming proteins. The machinery responsible for this function has been disrupted through CmdT, which changes viral mRNA so that it is unable to undergo translation, which would lead to structural proteins.
CmdT’s function is highly specific. The enzyme facilitates the bonding of adenosine diphosphate ribose to viral mRNA molecules, which are important in the process of protein construction. Such modification of mRNA structures is known as adp-ribosylation, a form of PTM. Since rabid protogenic systematically targets CmdT for mRNA, such tagging encapsulates the mismatch within small ribonucleic structures to facilitate the synthesis of proteins. Ultimately, CmdT uses a similar approach to infect cellular structures by disrupting a virus’s ability to generate all of its internal components, thus ceasing its replication inside bacterial cells.
How CmdT Shuts Down Translation
In their research, scientists found that CmdT proteins specifically bind to the 3′ end of mRNAs and, therefore, modify the poly-A tail region, which is essential in stabilizing mRNA and initiating mRNA translation. If the ribosome binding site is modified in this way by the process known as ADP-ribosylation, translation will not occur due to the inability of the viral mRNA to bind to the ribosome.
It is this targeting specificity that also makes CmdT efficient as well. CmdT acts on the viral mRNA by targeting the poly-A tail, whereby the bacterial mRNA is only minimally targeted. This untargeted inhibition enables the bacteria to better withstand a viral invasion while at the same time permitting the host to perform its routine cellular processes.
Experimental Insights: From Protein Blots to RNA-Seq
The researchers solving the problem were not content with one method to determine the details of CmdT’s action but used experimental approaches. By way of protein immunoblotting (Western blotting), CmdT present in bacterial cells was also confirmed. This technique enabled them to see the protein’s production and modification when the virus attacks a cell.
Then, the research group performed radiolabel incorporation assays to assess viral protein synthesis throughout an infection. By tagging amino acids with radioactive isotopes, they were able to show the extent of viral protein production in the presence of CmdT. Their studies indicated a significant loss of synthesizing viral proteins after CmdT was introduced, validating its function in translation inhibition.
To progress toward explaining the role of CmdT as an RNA, the researchers performed RNA-sequencing (RNA-seq) and ribosome profiling (RIP-seq). These methods were also helpful in determining what mRNA was translated and what was not translated. In the presence of CmdT, it was observed that there was a drastic under-representation of viral mRNAs in ribosome-associated fractions, which implied these mRNAs were not being translated. This gave clear evidence that CmT was successful in preventing the viral mRNA’s access to the ribosomes.
CmdT’s Role in Phage Resistance
The investigation further examined the origin of CmdT’s development within the context of an evolutionary arms race. Bacteria and phages are in a constant struggle to out-evolve one another. Thereafter, to see how CmdT evolved with this development, the researchers started experiments to advance the evolutionary processes of phages in bacteria that expressed CmdT.
Over several cycles of evolution, however, the phages gained some mutations, which helped them to at least partially bypass the defenses of CmdT, highlighting the dynamic interplay between bacterial defenses and phage countermeasures. Nevertheless, CmdT still offered considerable protection even with these modifications, which illustrated the effectiveness of CmdT as an antiviral strategy.
A New Layer of Bacterial Defense
This finding adds to the already well-known defenses present in bacterial immune systems, such as the CRISPR-Cas system. If CRISPR is used to cut the viral DNA, CmdT goes a step further by attacking the viral RNA. The exploitation of blocking translation at the mRNA level shows a unique way in which bacteria can resist phage attacks.
There is also room for more research based on the specificity and efficiency of CmdT. Could it be possible to devise or improve similar ADP-ribosyltransferases for use as antiviral or antibiotic-resistant treatments in practice?
Applications and Future Directions
Detection of CmdT as an mRNA-targeting defense mechanism encourages further studying the bacterial immune system. Researchers are likely to learn more about the natural resistance of bacteria to viral infection, which can help develop new antimicrobial agents, especially in phage therapy used to treat bacterial infections. Also, analyzing the adaptation of bacteria against phage CmdT could help design more efficient and long-lasting therapeutic phages. Understanding how phages resist being made irreversible allows researchers to design phages that will be useful even before the irreversible stage of evolution is reached.
Conclusion
The work done by Vassallo, Doering, and Laub reveals an interesting new mechanism of bacterial defense against viruses. CmdT, a bacterial ADP-ribosyltransferase, also prevents the translation of the viral mRNA and hence stops the replication of phages. This finding not only enhances the comprehension of the molecular rivalry that exists between bacteria and phages but also could lead to practical applications in the future. With the progress of knowledge regarding bacterial immune systems, such findings will be instrumental in devising strategies to combat viral infections and antibacterial drug resistance.
Article Source: Reference Paper | Reference Article | Code used for RNA-seq and in vivo RIP–seq analysis is available at Zenodo.
Disclaimer:
The research discussed in this article was conducted and published by the authors of the referenced paper. CBIRT has no involvement in the research itself. This article is intended solely to raise awareness about recent developments and does not claim authorship or endorsement of the research.
Follow Us!
Learn More:
Anchal is a consulting scientific writing intern at CBIRT with a passion for bioinformatics and its miracles. She is pursuing an MTech in Bioinformatics from Delhi Technological University, Delhi. Through engaging prose, she invites readers to explore the captivating world of bioinformatics, showcasing its groundbreaking contributions to understanding the mysteries of life. Besides science, she enjoys reading and painting.