In an extraordinary feat of chemical synthesis, chemists from Scripps Research have produced 25 picrotoxane molecules. This groundbreaking work combines cutting-edge computational modeling and innovative laboratory techniques to navigate the complex pathways of these functionally dense molecules. Their findings, published in Nature, not only shed light on picrotoxane chemistry but also push the boundaries of computer-aided synthesis planning (CASP).
Picrotoxin structure is a combination of several components classified as cyclic and alkaloid. These compounds exhibit a pharmacological profile that makes them effective as GABA-channel modulators. Picrotoxanes are chemobioactive compounds that bind to the GABAA receptor, which performs the function of a protein pore in neurons. Once bound, these compounds block the ion pore receptor, and this is where the structural polysilicon complexity becomes visible. However, for several years, the active recombination of these entities has proven more difficult than anticipated. This team has unlocked a more efficient pathway to these elusive targets by combining advanced computational techniques and strategic chemical design.
Computational Chemistry Meets Experimentation
One of the major innovations of this work is the addition of computational modeling into the synthetic process. The team created a DFT-based framework to model reaction selectivity and assist in synthetic decision-making. This could also allow them to analyze essential precursors computationally before pursuing any experimental routes, hence saving time and resources.
A promising step forward was the rational design modeling to investigate the relative rates of HAT and β-scission reactions. The researchers constructed a regression model that used two core parameters – the NBO charge on oxygen atoms and the interatomic distance between oxygen and carbon – and was able to predict the outcomes of picrotoxane intermediates.
The predicted model demonstrated a very close match when compared computationally and experimentally as far as selective prediction was concerned, such as a 4:1 HAT to β-scission ratio that was expected for some epoxides.
Innovative Synthetic Strategy
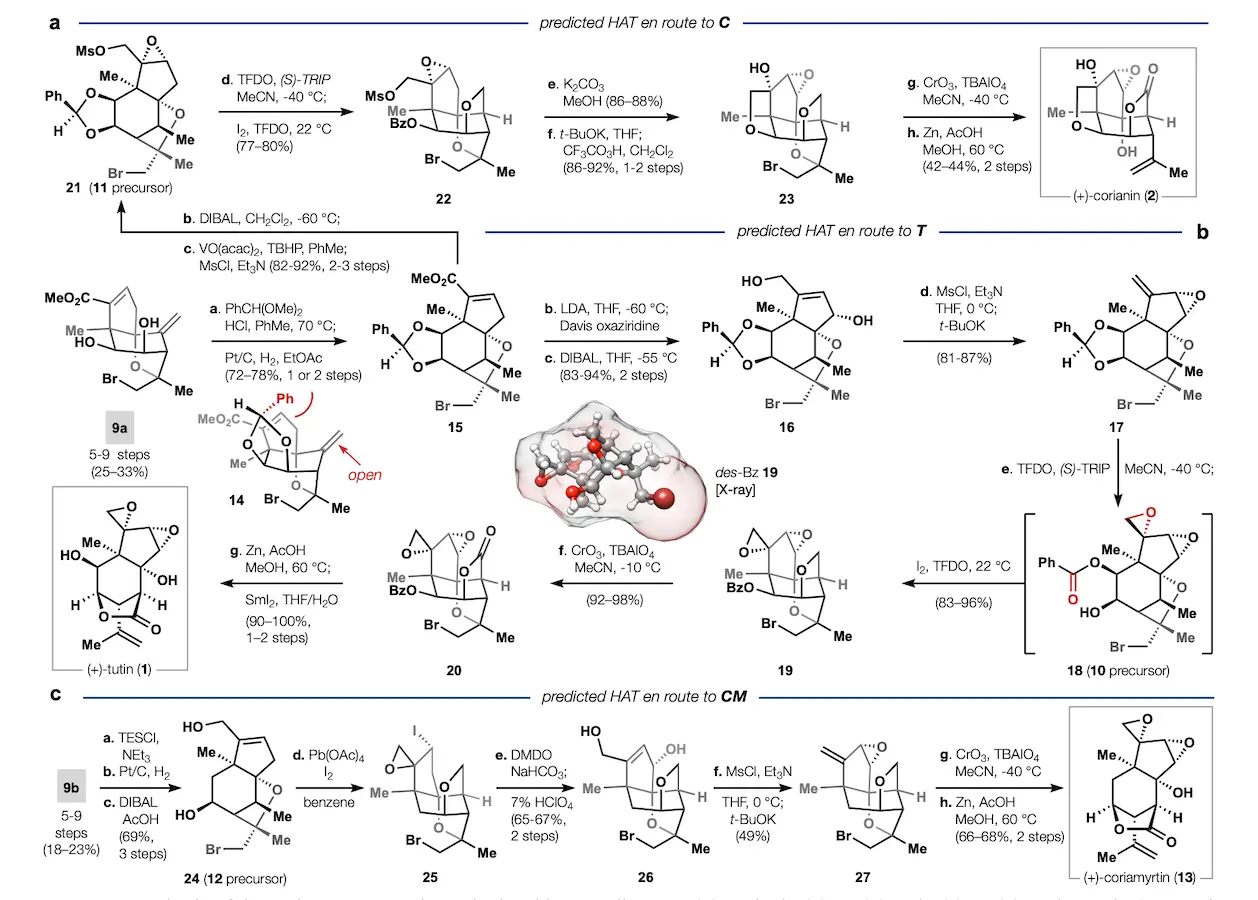
The synthesis strategy was modified in a manner that maintained high intermediate precision selectivity. For example, after the team begins with compound 9a, the common precursor, they then proceed to carry out transmetalation and convert it into a benzylidene acetal. This step had several important aspects, including:
- Protecting key functional groups,
- Favoring HAT over competitive pathways like β-scission,
- Enhancing regio- and stereoselectivity.
Subsequent steps included:
- Selective hydrogenation to yield intermediate 15.
- Oxidations and reductions to introduce functionality, with careful control of stereochemistry.
- Intramolecular substitutions to form epoxides, which set the stage for critical 1,5-HAT reactions.
The sequence culminated in the efficient synthesis of natural products like (+)-tutin, (+)-corianin, and (+)-coriamyrtin. For each target, the team optimized steps to maximize yields and minimize byproducts, achieving overall yields of 3-19% across 12-20 steps—a significant improvement over traditional approaches.
Key Successes: The Power of Prediction
The computational model facilitated every step individually as well as aided in the devising of a larger strategy but on the internal level. By way of example, the team highlighted how subtle changes in the B-ring substitution patterns of intermediates impacted dihedral angles φ and ψ. These angles determined the preference for HAT versus β-scission. A thorough understanding of those angles would have been more or less an impossible task without the aid of computational tools.
The most noteworthy accomplishments include the proposed and subsequent 1,5-HAT pathway for diepoxide intermediates critical for synthesizing numerous picrotoxanes. The regio- and stereochemical accuracy attained in these reactions was remarkable, and they point to the collaborative integrative nature of computation and experimentation.
Implications and Future Directions
The existence of 25 picrotoxanes demonstrates the capability of a computer-aided synthesis planning (CASP) system. They underscore the importance of comprehensively explaining and defining a complex target and building structure reactivity relationships when working with a target area that is as large as picrotoxanes. While traditional approaches often rely on trial and error or extensive precedent in the literature, CASP enables chemists to predict outcomes in uncharted territory.
In addition to the synthesis, the work described here offers prospects for studying the biological activity of picrotoxane derivatives. Many synthesized congeners do not have the labile C11 lactone, which may increase their stability and use as ion channel blockers. The team’s strategy also demonstrates the wider usefulness of CASP in speeding up the process of finding new molecules that possess medicinal significance.
Conclusion
The effort by researchers towards the total synthesis of 25 picrotoxanes can be regarded as a milestone in the field of synthetic chemistry. Through a fusion of computed predictions with entirely new experimental tools, they have succeeded in not only traversing the complex chemical space of picrotoxanes but also raising the bar of CASP to a new level in the synthesis of intricate molecules. This work shows how computational methods can provide the necessary assistance to human ingenuity to overcome challenges that once seemed impossible and open avenues for stronger breakthroughs in both chemistry and medicine.
Article Source: Reference Paper Abstract | Full Paper: ChemRxiv | Reference Article.
Disclaimer:
The research discussed in this article was conducted and published by the authors of the referenced paper. CBIRT has no involvement in the research itself. This article is intended solely to raise awareness about recent developments and does not claim authorship or endorsement of the research.
Follow Us!
Learn More:
Anchal is a consulting scientific writing intern at CBIRT with a passion for bioinformatics and its miracles. She is pursuing an MTech in Bioinformatics from Delhi Technological University, Delhi. Through engaging prose, she invites readers to explore the captivating world of bioinformatics, showcasing its groundbreaking contributions to understanding the mysteries of life. Besides science, she enjoys reading and painting.