Imagine a future where deadly bacterial infections can be easily defeated with custom-designed antimicrobial peptides (AMPs). Thanks to artificial intelligence (AI), that future might not be so far away! A team of researchers from Shandong University has made a groundbreaking discovery using AI to generate powerful new AMPs. Their work, published in Science Advances, showcases how a latent diffusion model can design diverse and highly effective peptides to combat antibiotic resistance.
Why Do We Need New Antimicrobials?
For many years, antibiotics served as life-saving goliaths, but their abuse has given birth to bacteria that are resistant to drugs. By 2050, it is predicted that death from drug-resistant infections may even outnumber cancer mortality rates – how horrible! The developing and discovery processes of drugs are always lengthy and costly, so why not try short AMPs? These short peptides attack bacteria in ways that make resistance less likely. The catch? Finding effective AMPs has been like searching for a needle in a haystack—until now.
AI to the Rescue: How It Works
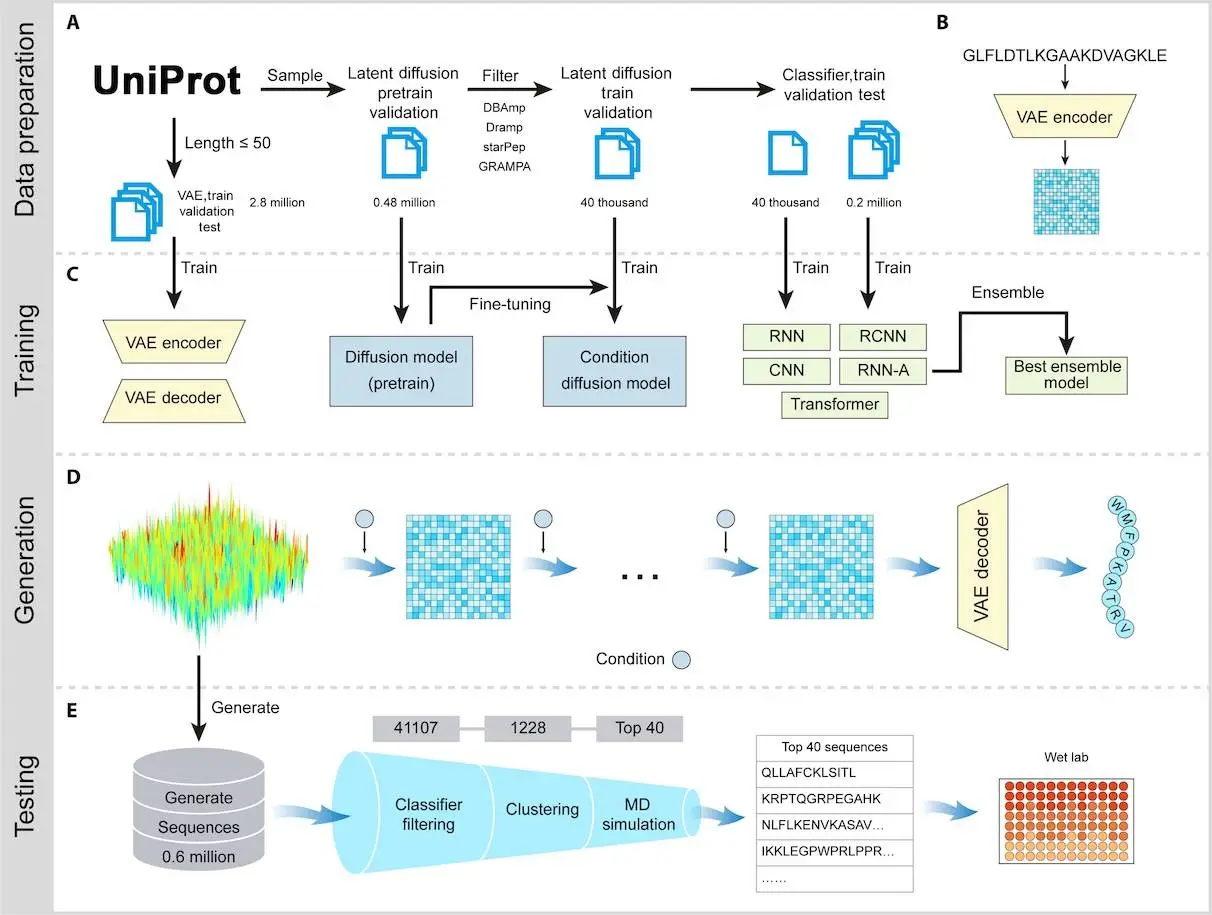
The research team has developed a latent diffusion model, which is a type of generative AI that incrementally perfects the sequence of the peptides to come up with unique and incredibly powerful AMPs. Most peptides are rediscovered using the traditional method. This AI system eliminates the need to use discovered or known peptides; instead, it creates compounds with high diversity and effectiveness unlike any other in existence.
The process is pretty cool:
- Generating Peptides – The AI churns out thousands of candidate sequences.
- Filtering the Best Ones – Advanced computational screening selects the most promising peptides.
- Lab Testing – The top contenders are synthesized and tested for real antimicrobial power.
The Results?
Of the 40 synthesized peptides, 25 showed strong antibacterial or antifungal activity. The standouts:
- AMP-29: A fungal fighter showed potent activity against Candida glabrata and even worked in live animal infection models.
- AMP-24: A bacterial assassin that tackled drug-resistant Acinetobacter baumannii, a major problem in hospitals worldwide.
These findings are extremely encouraging. AI-designed peptides not only demonstrate strong antimicrobial activities but also have higher structural diversity compared to conventionally derived ones. This is exhilarating as it sets the stage for creating AMPs that can tackle more than one pathogen type.
How Do These Peptides Work?
Instead of targeting certain bacterial processes, AMPs attempt to damage the membrane (physical means) of microbial cells, which, unlike antibiotics, is much harder to work around. Molecular dynamics simulations have shown that bacterial and fungal membranes are actively destroyed by the designed AMPs with the help of “chaos-inducing” destruction, which is fatal to the fungus/bacteria.
Furthermore, these peptides were demonstrated in vitro to significantly impact the structural integrity of bacterial and fungal cells. Transmission electron microscopy showed that membrane substrates were destroyed by AMP-24 and AMP-29, culminating in cell destruction. This is encouraging because it suggests these AMPs may constitute a new class of antibiotics that work without resistance.
Less Resistance, More Hope
What stands out the most? The low chance of resistance. Unlike other antibiotics, which spawn resistant strains in their wake, AMPs like AMP-24 and AMP-29 showed very little resistance development under lab conditions, which is amazing in the world of superbugs.
Scientists exposed the bacteria and fungi samples to the peptides at doses low enough not to kill to check if resistances were developed over subsequent generations. To their surprise, AMP-24 and AMP-29 had the lowest reduction in activity of the two, which makes it reasonable to believe that they can be used to treat infections for extended periods without significant resistance developing.
Conclusion
This experiment verified the theory that AI could innovate drug development, but there is so much left to be uncovered. Further work will center on making AMPs safe and effective for human use and other ailments, such as cancer or metabolic diseases, and so on.
The intersection of AI and biotechnology is producing jaw-dropping innovations, and this AI-powered AMP discovery is one of the most exciting yet. As researchers continue to fine-tune these models, we might just have the ultimate weapon against drug-resistant infections.
Article Source: Reference Paper | The codes supporting the findings of the study are available on GitHub.
Disclaimer:
The research discussed in this article was conducted and published by the authors of the referenced paper. CBIRT has no involvement in the research itself. This article is intended solely to raise awareness about recent developments and does not claim authorship or endorsement of the research.
Follow Us!
Learn More:
Anchal is a consulting scientific writing intern at CBIRT with a passion for bioinformatics and its miracles. She is pursuing an MTech in Bioinformatics from Delhi Technological University, Delhi. Through engaging prose, she invites readers to explore the captivating world of bioinformatics, showcasing its groundbreaking contributions to understanding the mysteries of life. Besides science, she enjoys reading and painting.