Enzymes can mediate discrete chemical reactions with sub-angstrom precision by employing complex, polar active sites to ensure a precise and effective response. Using the traditional catalytic triad and the oxyanion hole of serine hydrolases as a model system, researchers from the University of Washington, USA, attempted to address this difficulty. ChemNet is a recently discovered ensemble generation tool that allows researchers to examine active site geometry and preorganization at each step of the reaction. Researchers employed RFdiffusion to produce proteins housing catalytic sites of diverse geometry and increasing complexity. It has been found that serine hydrolases catalyze ester hydrolysis with great efficiency, in accordance with design models. Screens containing as little as 20 patterns can be used to identify these hydrolases, which differ from natural serine hydrolases in their folds. In addition to structural and mutational investigations of native enzymes, the de novo building approach sheds light on the geometric factors that govern catalysis. This offers a guide for creating complicated enzymes that catalyze multi-step reactions and serine hydrolases of industrial relevance, hence increasing the efficiency of these enzymes.
Introduction
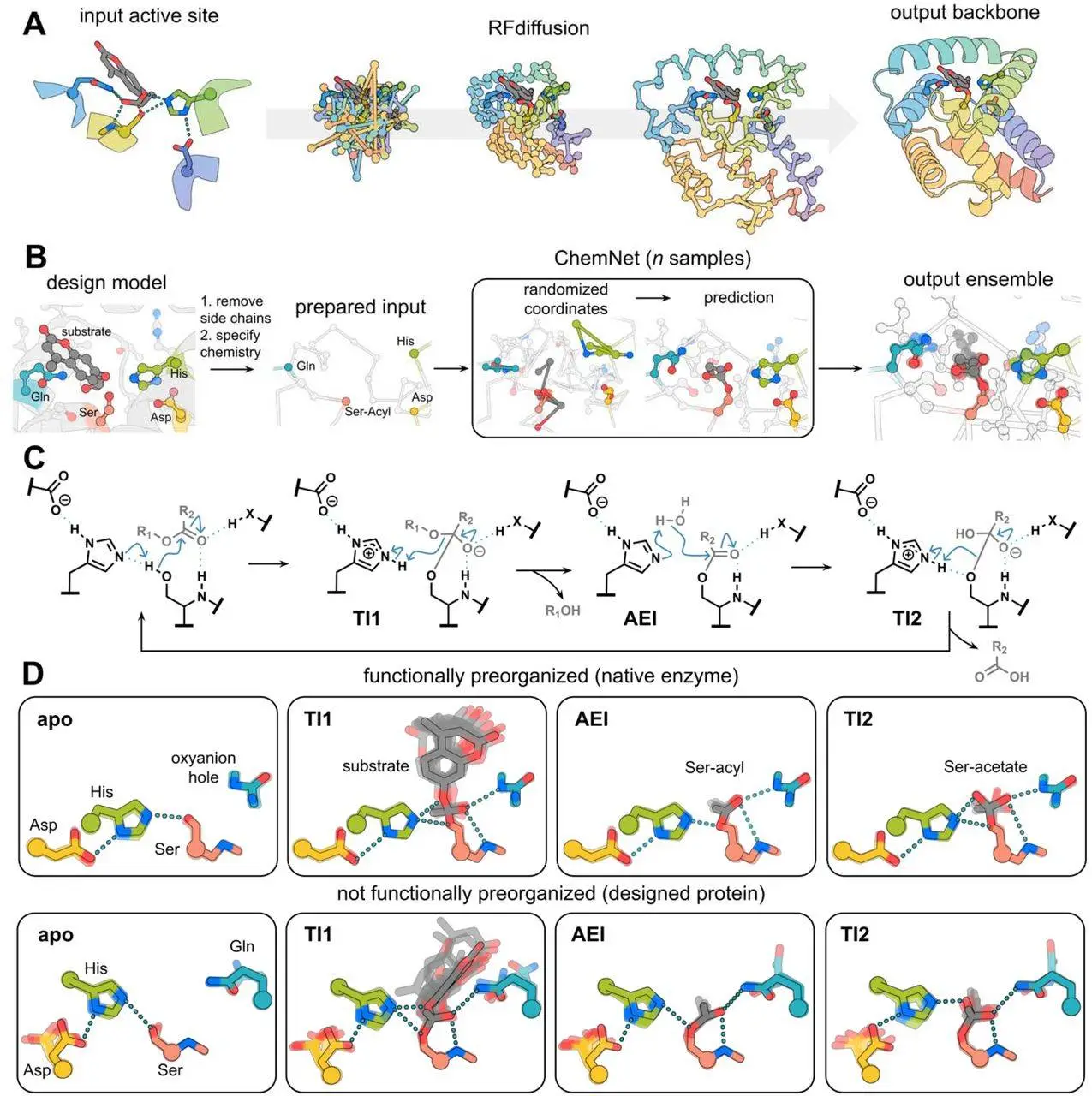
In mild water-based conditions, enzymes are excellent catalysts that quicken reaction speeds. Determining the location of the active site in pre-existing scaffolds and organizing catalytic residues around the reaction transition state (theozyme) are the main objectives of enzyme design, a long-standing endeavor in computational protein design. The activities of many designed enzymes are, however, limited by fixed backbone scaffolds, which limit the accuracy of catalytic geometry. An additional obstacle is the precise atomic preorganization of the active site, particularly in multistep reaction processes that stabilize several transition states and intermediates. Current in silico techniques to assess design preorganization are usually limited to one reaction state and have minimal computational cost or accuracy. Novel techniques are required for the synthesis of protein backbones that are especially suited to a given active site and for evaluating their structural compatibility across the course of the catalytic cycle in order to facilitate the precise design of multistep enzymes.
Key Findings of the Study
- Researchers reasoned that building blocks of proteins to scaffold a particular active site and evaluate compatibility across a specified reaction coordinate might be achieved by designing proteins from scratch using breakthroughs in deep learning for protein design and structure prediction. Researchers wanted to apply a similar strategy to build enzymes starting from geometric descriptions of an active site. Recent developments in structuring functional sites using RFdiffusion have produced better in silico and experimental success rates across a range of design objectives. By modeling structural ensembles of catalytic intermediates, scientists aimed to take advantage of developments in deep learning-based prediction of protein-small molecule complexes to evaluate preorganization and functional connections in each phase of the catalytic cycle.
- Active serine hydrolases have been designed using computational characterization and structural analysis, however, de novo design has not proven fruitful with these methods. Proteins with activated cysteines and serines are unable to catalyze turnover, despite much investigation. To find the active site in a collection of NTF2 scaffolds produced by deep learning, an initial design campaign was carried out. On substrates made of activated ester, however, experimental characterization showed activated serines but no catalytic turnover, indicating that crucial components required for catalysis were absent.
- ChemNet was utilized by the researchers to create structural ensembles for a set of native and previously created serine hydrolases for each of the four chemical stages. The results of these computations demonstrated that native hydrolases have a significantly higher degree of preorganization than previously developed systems. At the same time, there are often large changes in the ensembles at several steps in planned systems, in native systems the catalytic residues at each step sample a relatively small number of conformations in which the important hydrogen bonding interactions are preserved. It is anticipated that catalysis will be compromised by the absence of preorganization of the planned active sites since the reaction rate should be proportionate to the percentage of the enzyme in the active state.
- The structures of super and win were designed using X-ray crystallography, and the results showed that their Cα RMSDs were lower than those of the design models. With the sidechain conformations of the catalytic residues in atomic agreement for both super and win, the structures likewise demonstrated good agreement between the planned and experimental structures. A water molecule in the superactive site establishes hydrogen bonds with the oxyanion hole contacts, probably in an attempt to mimic the ester substrate’s carbonyl oxygen. At the catalytic center of win, an acetate molecule forms hydrogen connections with the oxygen of T99, the histidine acid/base residue H17, the Oγ and backbone amide nitrogen of the catalytic serine S142, and other important hydrogen bonds in the catalytic cycle.
- Using RFdiffusion and ChemNet ensemble analysis, the de novo buildup strategy seeks to guarantee preorganization and design accuracy, allowing for the direct development of hypotheses in addition to the more conventional methods based on structural analysis and mutation of highly evolved native enzymes.
Applications of ChemNet
- Enzyme design has long faced difficulty in evaluating design compatibility across the catalytic cycle. The deep neural network ChemNet, which the researchers have demonstrated can quickly create ensembles for a variety of chemical intermediates, may directly evaluate preorganization and offer structural insights that would otherwise necessitate time-consuming structural investigations. For instance, ChemNet uncovered widespread off-target conformational alterations in the acyl-enzyme, which may be the cause of several previously developed esterases’ inability to catalyze turnover.
- As ChemNet is stochastic, it offers ensemble views of the energy landscapes surrounding important reaction intermediates. The observation of agreement between experimental success rates and ChemNet preorganization indicates that such ensemble creation will be broadly beneficial for enzyme design in the future.
Conclusions
In spite of restrictions on sidechain location and active site geometry, the design of a serine hydrolase has greatly increased catalytic efficiency. In contrast to natural evolution, the generative deep learning design approaches have discovered fresh approaches to design problems. The developed catalysts’ folds differ from those of serine hydrolases found in nature, illustrating how generative deep learning can provide novel solutions. Earlier attempts to create catalytic triad-based designs have not been successful in achieving multiple turnovers, and certain designs have encountered difficulties in regulating the geometry of histidines, which has limited the activation of leaving groups and water. These restrictions are circumvented by de novo backbone production that uses RF diffusion to build outward from a designated active point. ChemNet has the potential to be used in the near future to build a wide range of novel catalysts because of its capacity to accurately locate several catalytic groups and evaluate the organization of the active site throughout complex processes.
FAQ
The oxyanion hole and catalytic triad of natural serine hydrolases catalyze ester hydrolysis, which has been used for decades as a model reaction for computational enzyme design. This reaction is catalyzed by one of the most well-documented enzymatic processes.
The catalytic cycle comprises four distinct steps: The first tetrahedral intermediate (TI1) is formed when the substrate initially attaches to the apoenzyme (apo), deprotonating the catalytic serine and attacking the ester’s carbonyl atom. Second, the active site remains serine covalently bound to the substrate’s acyl group (acyl-enzyme intermediate, AEI) as a result of the catalytic histidine protonating the leaving group oxygen and encouraging its departure. Third, a second tetrahedral intermediate (TI2) is produced when the histidine deprotonates a water molecule, which then attacks the AEI. Ultimately, the catalytic cycle is completed and the free enzyme is reconstituted by the histidine-mediated protonation of serine and release of the acyl group, which resolves this intermediate.
Throughout, two hydrogen bond donors that make up the oxyanion hole stabilize negatively charged transition states and intermediates. The interaction between aspartate or glutamate, the last residue in the triangle, and histidine causes a perturbation in its pKa, which regulates its acid/base function.
Article Source: Reference Paper
Important Note: bioRxiv releases preprints that have not yet undergone peer review. As a result, it is important to note that these papers should not be considered conclusive evidence, nor should they be used to direct clinical practice or influence health-related behavior. It is also important to understand that the information presented in these papers is not yet considered established or confirmed.
Follow Us!
Learn More:
Deotima is a consulting scientific content writing intern at CBIRT. Currently she's pursuing Master's in Bioinformatics at Maulana Abul Kalam Azad University of Technology. As an emerging scientific writer, she is eager to apply her expertise in making intricate scientific concepts comprehensible to individuals from diverse backgrounds. Deotima harbors a particular passion for Structural Bioinformatics and Molecular Dynamics.










[…] Shaping the Future of Enzyme Catalysis: Advances in the Computational Design of Serine Hydrolases […]